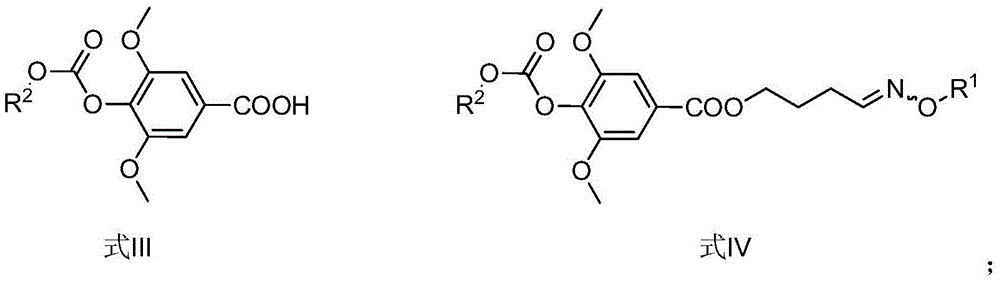
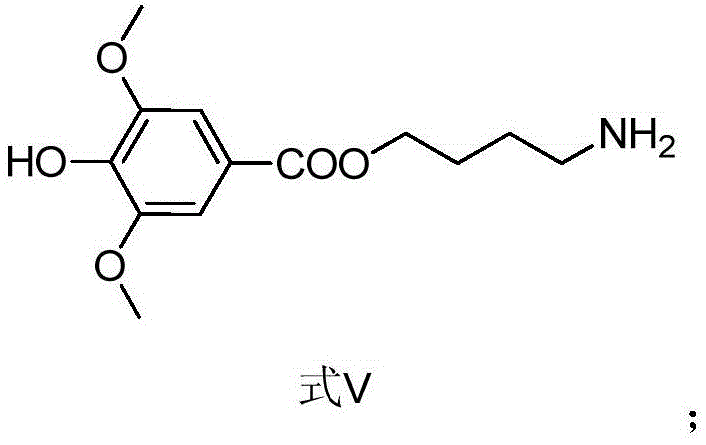
Method for synthesizing leonurine
A technology of leonurine and leonurine, which is applied in the field of leonurine synthesis, can solve the problems of low yield, poor economy and high production cost, and achieves the effects of high atomic economy, convenient operation and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
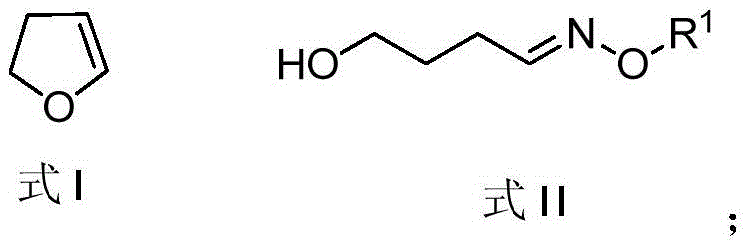
[0045] (1) Synthetic reaction of alkoxy oxime
[0046] Put the 250mL flask in an ice bath at 0°C, add 2M HCl (100mL), and then slowly add 2,3-dihydrofuran (10g, 140mmol) shown in formula I dropwise to the stirring In the above flask, continue to stir for 30 min after the dropwise addition, then take out the flask, and stir the reaction at room temperature for 2 hours; remove the solvent under reduced pressure, dissolve the resulting crude product in ethanol (100 mL), add pyridine (12.9 mL, 0.16 mol) and O -Methylhydroxylamine hydrochloride (12.53g, 0.15mol), heated to reflux for reaction, after 2h, remove ethanol under reduced pressure, dissolve with dichloromethane, wash with water, dry the organic phase with magnesium sulfate, filter, remove the solvent under reduced pressure A yellow liquid product 4-hydroxybutyl-O-methyloxime (16.5 g) was obtained, the structural formula of which is shown in Formula II.
[0047] The reaction formula of this step is shown in formula (1). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com