Method for detecting leonurine hydrochloride content in nephritis elimination tablet
A technology for the detection of motherwortine hydrochloride and its detection method, which is applied in the detection field of active ingredients of Chinese patent medicines, can solve problems such as the inability to guarantee the safety and effectiveness of motherwort compound preparations, and the lack of research on the determination method of motherwortine content, and achieves simple operation, high sensitivity, and heavy weight. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
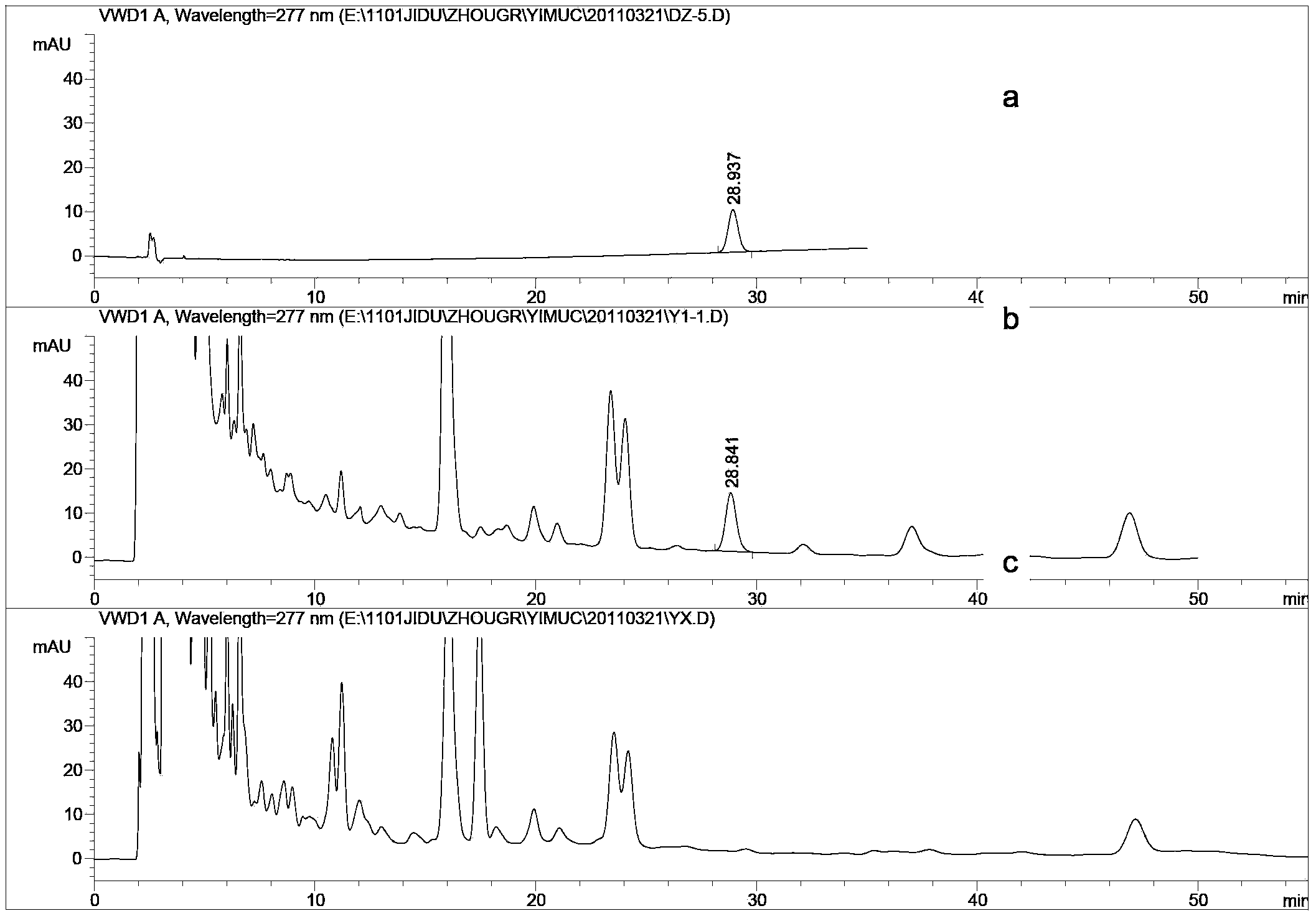
[0016] Experimental Example 1 Study on the Methodology of Determination of Leonurine Hydrochloride in Shenyanqing Tablets
[0017] 1 Instruments and test drugs
[0018] Instrument: Agilent1200 high performance liquid chromatography. Experimental drugs: Acetonitrile is chromatographically pure (MERCK), water is dipure water; Leonuri hydrochloride reference substance (Shanghai Dingrui Chemical Co., Ltd., purity ≥ 98%); Shenyanqing Tablets are prepared by Tasly Pharmaceutical Group Co., Ltd. according to the above method pilot test samples.
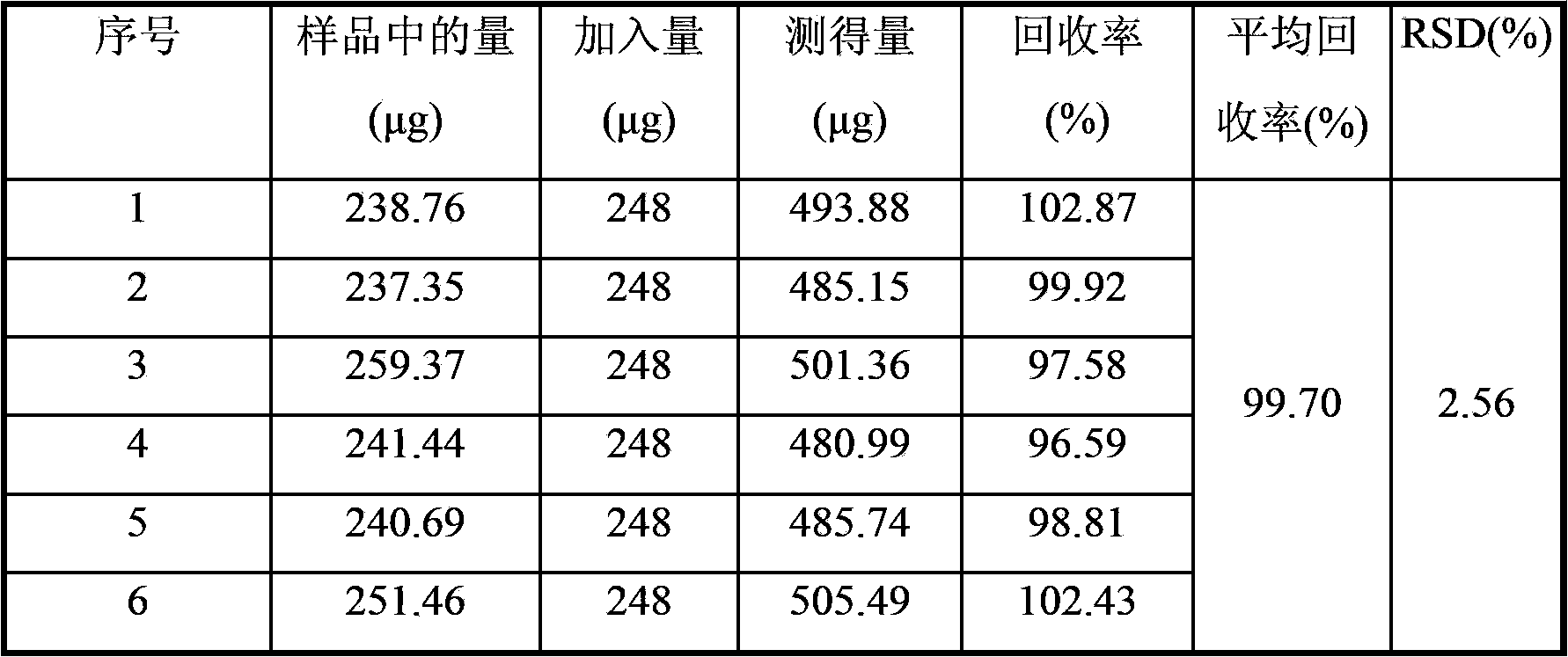
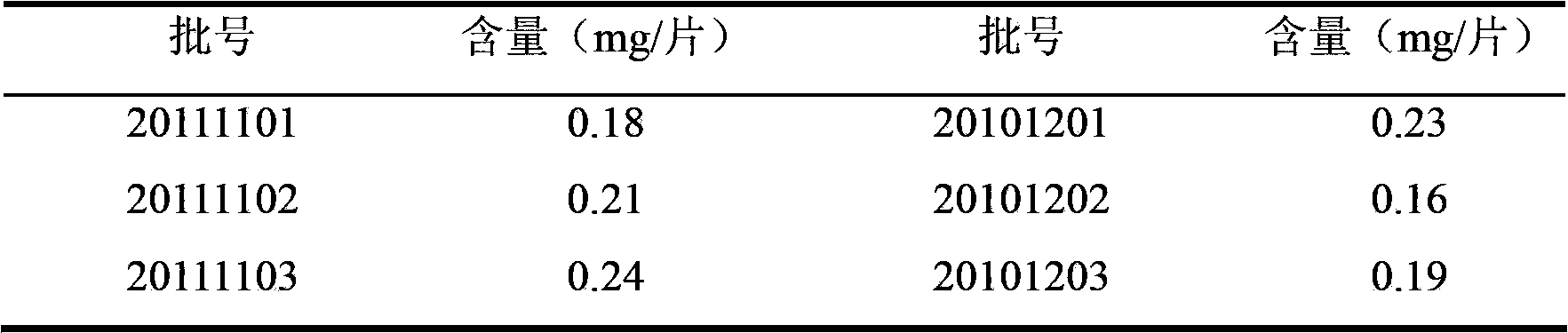
[0019] 2 Methods and results
[0020] 2.1 Chromatographic conditions
[0021] Chromatographic column: Agilent SB-C18 column (4.6×250mm, 5μm); flow rate: 1.0mL / min; column temperature: 30°C; mobile phase: acetonitrile-0.4% sodium octane sulfonate in 0.1% phosphoric acid solution (volume ratio 24 : 76); the detection wavelength is 277nm.
[0022] 2.2 Preparation of reference solution
[0023] Take an appropriate amount of motherwortine h...
Embodiment 1
[0046]1. Preparation of Shenyanqing Tablets Astragalus 400g Motherwort 400g, Imperata 200g Imperata 200g Qumai 200g Small Thistle 200g Shiwei 80g Burnet Charcoal 60g Rhubarb 200g Licorice 20g. Astragalus, add 6 times the amount of water to decoct for 1 hour, filter the decoction and concentrate to a thick paste, and vacuum dry to obtain the dry paste of Astragalus extract. Add 10 times the amount of 65% ethanol to reflux for extraction of rhubarb and small thistle, concentrate the decoction to a thick paste, and dry in vacuum to obtain a dry paste of rhubarb and small thistle extract. Add 6 times the amount of water to decoct the motherwort, Imperata rhizome, Liquor, Qumai, Shiwei, Burnet charcoal, and licorice. The decoction is concentrated to a relative density of 1.1 (60°C), add 3 times the amount of ethanol, and stand for 12 hours Above, filter, and recover ethanol from the filtrate to a thick paste, and vacuum-dry to obtain a dry paste of Qiwei extract. The above three d...
Embodiment 2
[0057] The preparation of Shenyanqing tablet and the qualitative determination of the effective components of Motherwort are the same as in Example 1.
[0058] Chromatographic conditions and system suitability test Octadecylsilane bonded silica gel was used as filler; acetonitrile-0.4% sodium octanesulfonate in 0.1% phosphoric acid solution (40:60) was used as mobile phase; detection wavelength was 260nm. The number of theoretical plates should not be less than 4000 based on the peak of Leonurine Hydrochloride.
[0059] Preparation of Reference Substance Solution Take an appropriate amount of Leonurine Hydrochloride reference substance, accurately weigh it, add 40% ethanol to make a solution containing 20 μg per 1 ml, and obtain it.
[0060] Preparation of the test solution Take the contents of this product under the weight difference item, grind or pulverize, mix, take 2.0g, accurately weigh, put in a stoppered conical flask, add 25ml of 50% ethanol, weigh, Sonicate for 35 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com