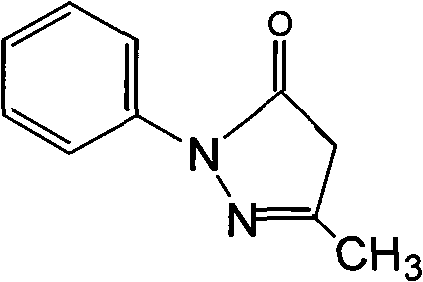
Edaravone injection and preparation method thereof
A technology of edaravone and injection, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, and medical preparations containing active ingredients. Water and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
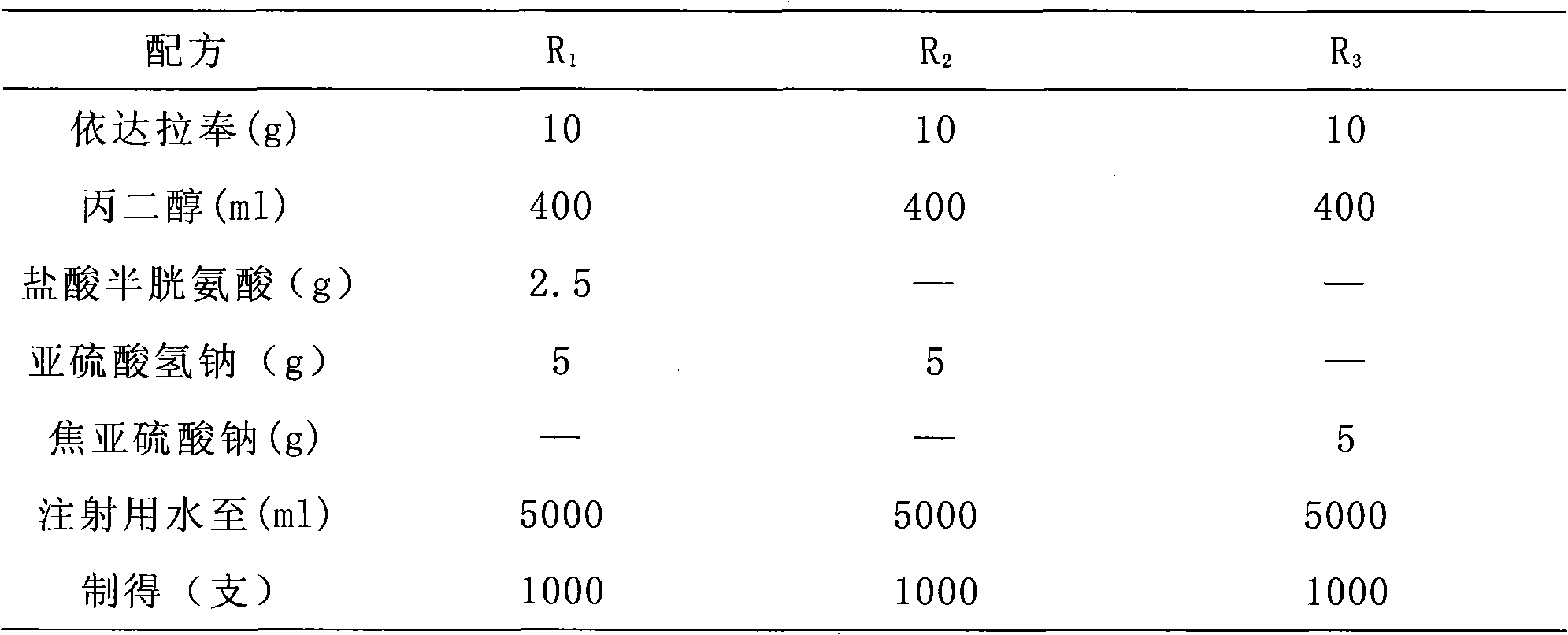
[0082] Embodiment 1: specification: 5ml: 10mg
[0083] formula:
[0084]
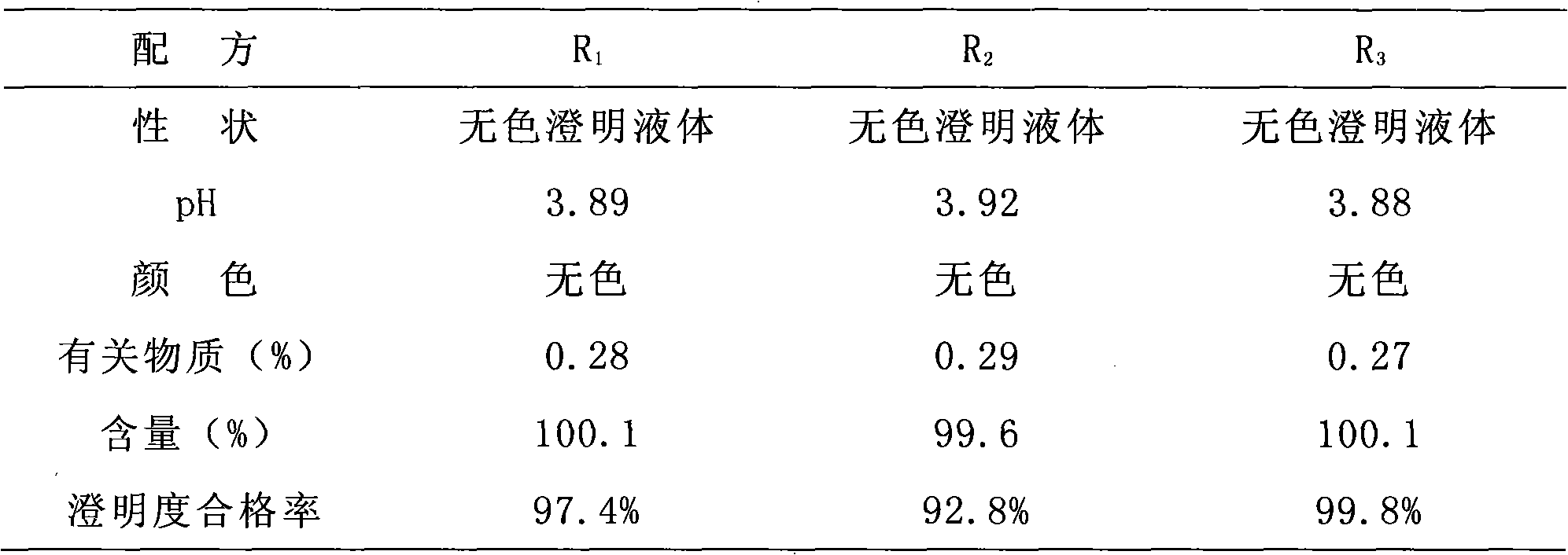
[0085] Take a propylene glycol hot water bath and heat to 60-70°C, add Edaravone, and stir until completely dissolved. Separately weigh sodium metabisulfite (0.1%) and pour it into a beaker, add water for injection and stir until completely dissolved. Slowly add the pre-dissolved antioxidant-containing solution to the edaravone propylene glycol solution while stirring, and then slowly add water for injection to 1 / 4 of the total volume while stirring. Then add activated carbon for needles (0.1%), warm and stir for 15 minutes, let stand, filter with suction, and remove carbon. Add the remaining water for injection. Adjust the pH to 4.0 with 0.1mol / L hydrochloric acid, check the intermediate, and fine-filter it with a 0.22μm microporous membrane until it is clear, fill it with nitrogen, fill it in an ampoule, and sterilize it by autoclaving at 115°C for 30 minutes. After three years, all inspections...
Embodiment 2
[0086] Embodiment 2: specification: 5ml: 10mg
[0087] formula:
[0088]
[0089] Take a propylene glycol hot water bath and heat to 60-70°C, add Edaravone, and stir until completely dissolved. Separately weigh sodium metabisulfite (0.15%) and pour it into a beaker, add water for injection and stir until completely dissolved. Slowly add the pre-dissolved antioxidant-containing solution to the edaravone propylene glycol solution while stirring, and then slowly add water for injection to 1 / 4 of the total volume while stirring. Then add activated carbon for needles (0.1%), warm and stir for 15 minutes, let stand, filter with suction, and remove carbon. Add the remaining water for injection. Adjust the pH to 4.2 with 0.1 mol / L hydrochloric acid, check the intermediate, and fine-filter it with a 0.22 μm microporous membrane until it is clear, fill it with nitrogen, fill it in an ampoule, and sterilize it by autoclaving at 115°C for 30 minutes. After three years, all inspecti...
Embodiment 3
[0090] Embodiment 3: specification: 20ml: 30mg
[0091] formula:
[0092]
[0093]Take a propylene glycol hot water bath and heat to 60-70°C, add Edaravone, and stir until completely dissolved. Separately weigh sodium metabisulfite (0.1%) and pour it into a beaker, add water for injection and stir until completely dissolved. Slowly add the pre-dissolved antioxidant-containing solution to the edaravone propylene glycol solution while stirring, and then slowly add water for injection to 1 / 4 of the total volume while stirring. Then add activated carbon for needles (0.1%), warm and stir for 15 minutes, let stand, filter with suction, and remove carbon. Add the remaining water for injection. Adjust the pH to 3.9 with 0.1mol / L hydrochloric acid, check the intermediate, fine filter with a 0.22μm microporous membrane until it is clear, fill it with nitrogen, fill it in an ampoule, and sterilize it by autoclaving at 115°C for 30 minutes. After three years, all inspections meet t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com