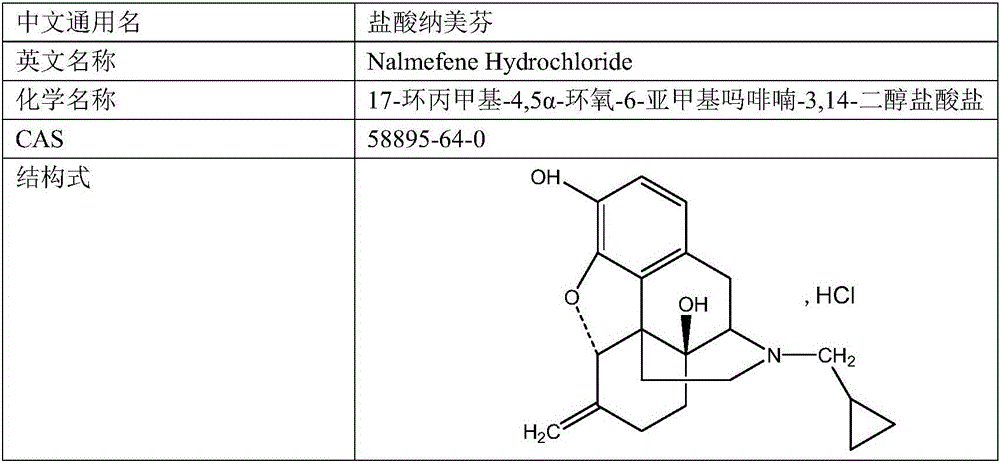
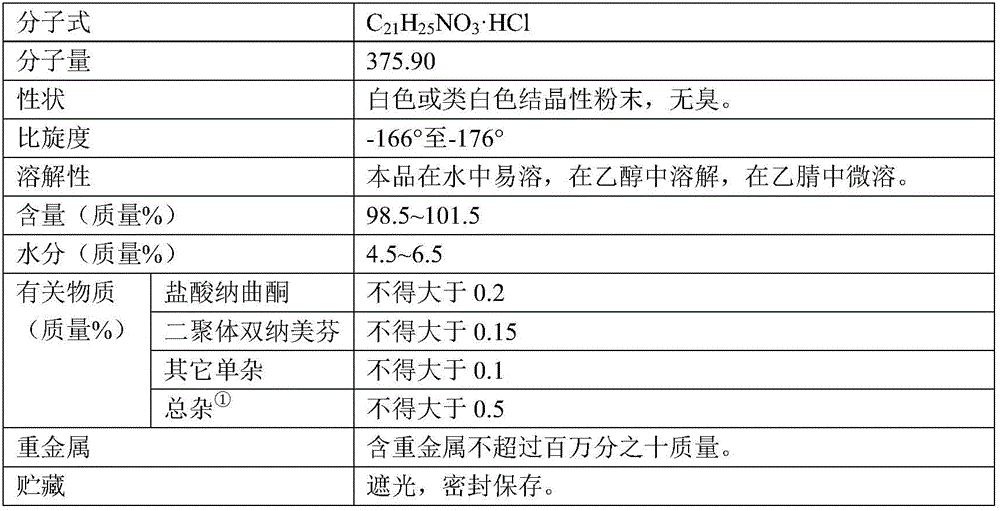
Nalmefene hydrochloride injection and preparation method thereof
A technology of nalmefene and injection, which is applied in the field of medicine, can solve problems such as increasing the content of single and total impurities in injections, affecting the activity of active ingredient nalmefene, and affecting the long-term stability of drugs, so as to achieve good long-term stability, Suitable for large-scale industrial production, easy to control the effect of automation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
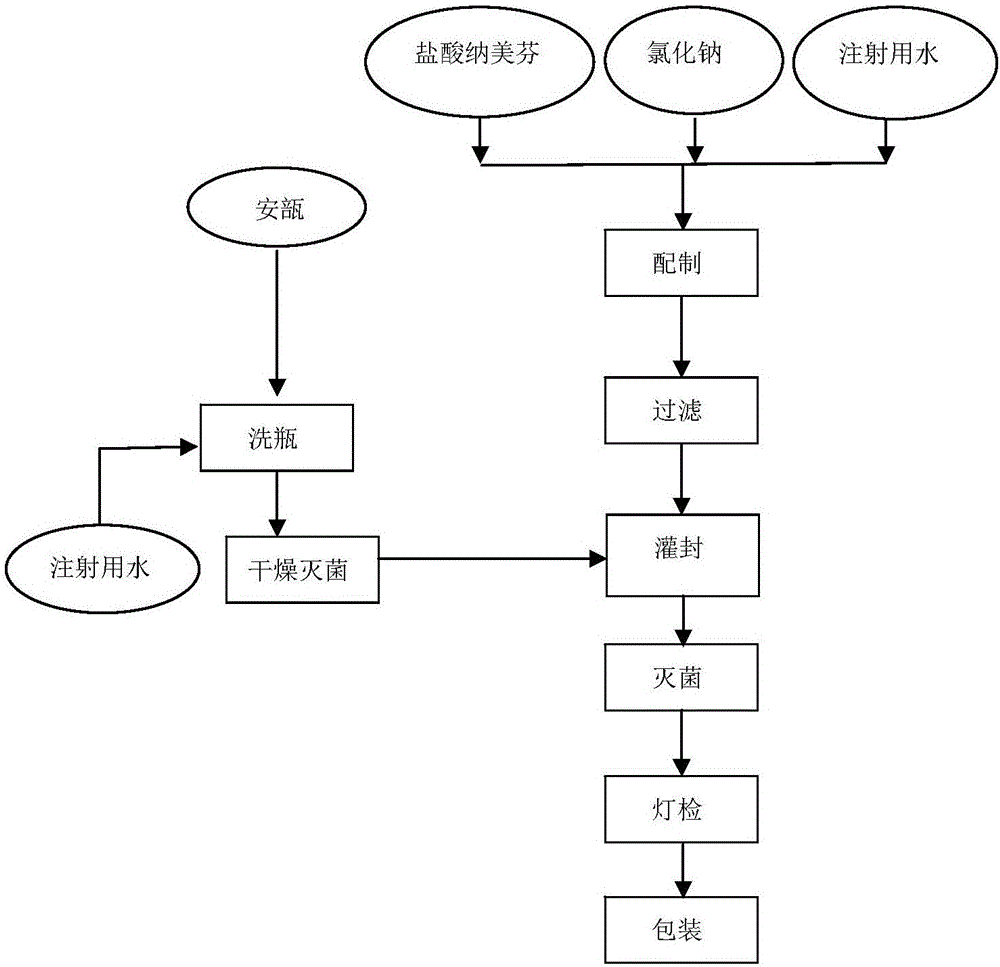
[0038] The nalmefene hydrochloride injection of the present embodiment is prepared by the method comprising the following steps (the process flow is as follows: figure 1 shown):
[0039] 1) Take water for injection and put it in the liquid preparation tank, feed nitrogen, add 4g of C 21 h 25 NO 3 The nalmefene hydrochloride crude drug of 20 mg / ml is stirred and dissolved to make a medicinal solution A with a concentration of 2mg / ml; in the medicinal solution A, add 360g of sodium chloride to dissolve completely to obtain the medicinal solution B;
[0040] 2) Under the protection of nitrogen, add activated carbon to the liquid B, stir and adsorb for 15 minutes, and then use a titanium rod to filter and decarbonize to obtain the liquid C; the dosage of activated carbon is 0.05mg / 100ml liquid A (ie 0.05% (W / V));
[0041] Rinse the titanium rod with an appropriate amount of water for injection, repeat 4 times, and add the washing water to the medicinal solution C;
[0042] 3)...
Embodiment 2
[0046] The nalmefene hydrochloride injection of the present embodiment is prepared by the method comprising the following steps:
[0047]1) Take water for injection and put it in the liquid preparation tank, feed nitrogen, add 10g of water in C 21 h 25 NO 3 The nalmefene hydrochloride crude drug of 900g is stirred and dissolved to make a concentration of 2mg / ml medicinal solution A; in the medicinal solution A, add 900g of sodium chloride to dissolve completely to obtain the medicinal solution B;
[0048] 2) Under the protection of nitrogen, add activated carbon (2.5g) to liquid B, stir and adsorb for 15min, and then use titanium rod to filter and decarbonize to obtain liquid C; the dosage of activated carbon is 0.05mg / 100ml of liquid A (i.e. 0.05% of liquid A (W / V));
[0049] Rinse the titanium rod with an appropriate amount of water for injection, repeat 5 times, and add the washing water to the medicinal solution C;
[0050] 3) Under the protection of nitrogen, add wate...
Embodiment 3
[0054] The nalmefene hydrochloride injection of the present embodiment is prepared by the method comprising the following steps:
[0055] 1) Take water for injection and put it in the liquid preparation tank, feed nitrogen, add 0.5g of C 21 h 25 NO 3 The nalmefene hydrochloride crude drug of 20 mg / ml is stirred and dissolved to make a medicinal solution A with a concentration of 2mg / ml; in the medicinal solution A, add 45g of sodium chloride to dissolve completely to obtain the medicinal solution B;
[0056] 2) Under the protection of nitrogen, the activated carbon added to the medicinal solution B was stirred and adsorbed for 15 minutes, and then decarbonized by filtering with a titanium rod to obtain the medicinal solution C; the dosage of activated carbon was 0.05mg / 100ml of the medicinal solution A (that is, 0.05 %(W / V));
[0057] Rinse the titanium rod with an appropriate amount of water for injection, repeat 3 times, and add the washing water to the medicinal solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com