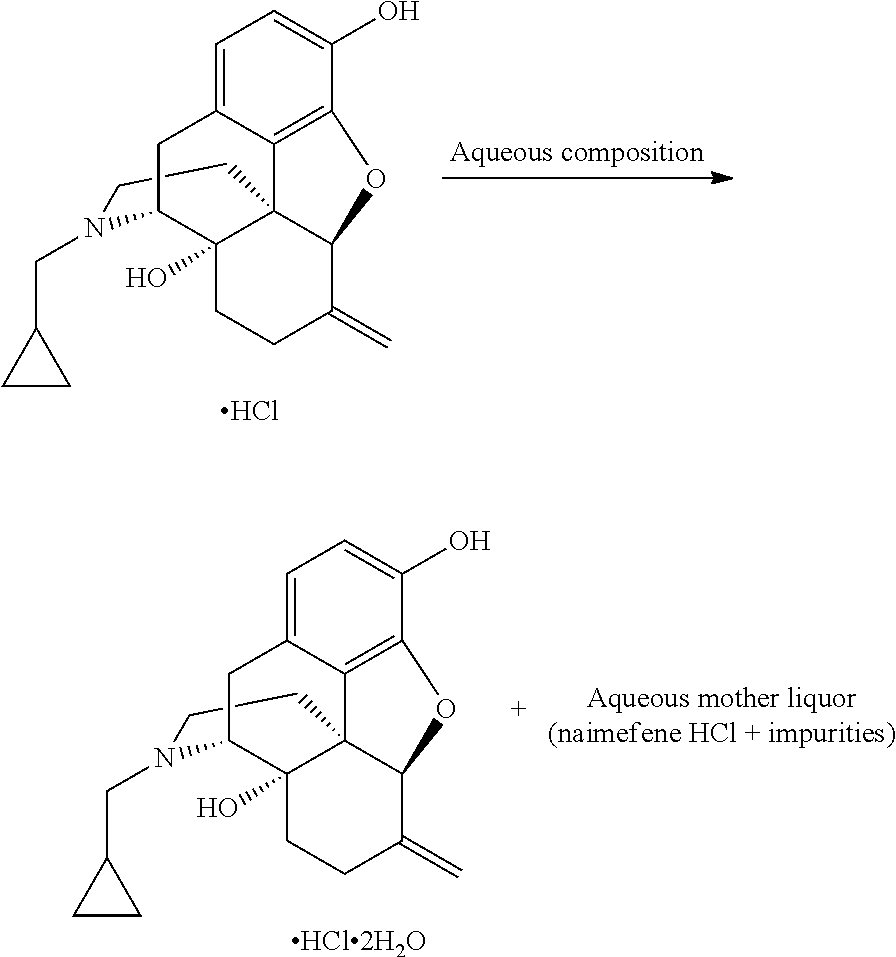
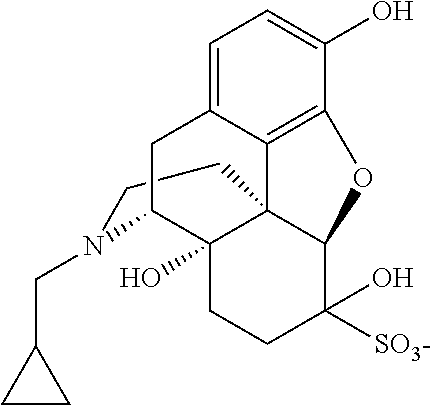
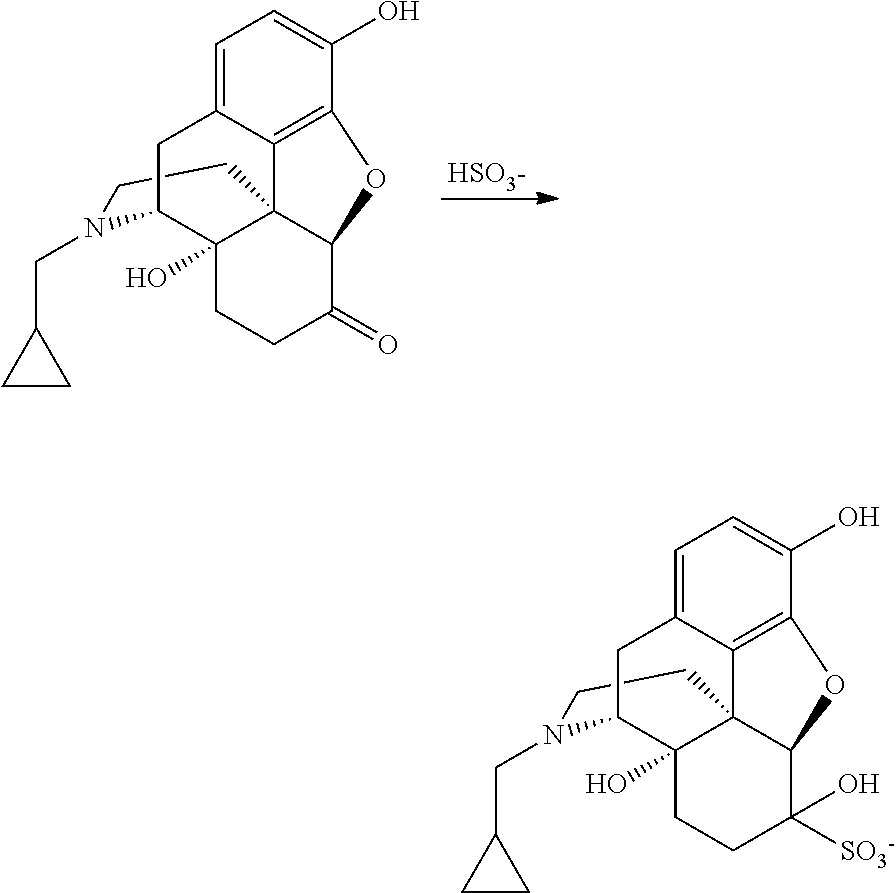
Process for recovery of nalmefene hydrochloride
a technology of nalmefene hydrochloride and recovery process, which is applied in the field of recovery process of nalmefene hydrochloride, can solve the problem that the synthesized precursor of nalmefene is difficult to remove selectively in the recovery process, and achieves the effect of improving the recovery process and improving the recovery process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Recovery of Nalmefene Hydrochloride from Aqueous Solution
[0092]Nalmefene HCl aqueous mother liquor (400 g); containing about 36 g of “residue of evaporation” with the following composition (% by HPLC area): nalmefene 92.08%, naltrexone 0.49%, methyldiphenylphosphine oxide 0.77%, triphenylphosphineoxide 4.0%; was treated with 0.34 g of sodium bisulfite and concentrated under vacuum distilling 323 g of solvent. The concentrated mixture was cooled down to 0° C. and maintained at this temperature overnight. The solid was filtered and washed with acetone (34 mL) obtaining 21 g of nalmefene HCl. HPLC analysis (% by area): nalmefene 98.92%, naltrexone 0.09%, methyldiphenylphosphine oxide 0.04%, triphenylphosphineoxide 0.16%.
example 2
Recovery of Nalmefene Hydrochloride from Aqueous Solution (Including Extraction with Organic Solvent)
[0093]Nalmefene HCl aqueous mother liquor (400 g); containing about 36 g of “residue of evaporation” with the following composition (% by HPLC area): nalmefene 92.08%, naltrexone 0.49%, methyldiphenylphosphine oxide 0.8%, triphenyiphosphineoxide 4.0%; was extracted with dichloromethane (2*50 mL). The aqueous layer was then concentrated under vacuum obtaining a suspension. Sodium bisulfite (0.3 g) was charged and the mixture was maintained at 60-65° C. for three hours. The suspension was cooled down to 0-5° C. and the solid was filtered and washed with dichloromethane (50 mL) and acetone (about 30 mL) obtaining 28 g of nalmefene HCl. HPLC analysis (% by area): nalmefene 99.36%, naltrexone 0.12%. Methyldiphenylphosphine oxide and triphenylphosphineoxide below detection limit.
example 3
Recovery of Nalmefene Hydrochloride from Aqueous Solution (Including Extraction with Organic Solvent)
[0094]Nalmefene HCl aqueous mother liquor (145 kg); containing about 16 kg of “residue of evaporation”, with the following composition (% by HPLC area): nalmefene 90.97, naltrexone 0.23%, methyldiphenylphosphine oxide 1.1%, triphenylphosphineoxide 4.9%; was extracted with dichloromethane (2*30 L). The aqueous layer was then concentrated under vacuum distilling 128 kg of solvent. The suspension was diluted with water (7 kg). Sodium bisulfite 0.15 kg was added and the mixture maintained at 60-65° C. for four hours. The suspension was cooled to 0-5° C. and stirred for four hours. The solid was filtered and washed with dichloromethane (15 L) and acetone (15 L) obtaining 12.8 kg of nalmefene HCl. HPLC analysis (% by area): nalmefene 99.12%, naltrexone 0.05%. Methyldiphenylphosphine oxide and tri-phenylphosphineoxide below detection limit.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com