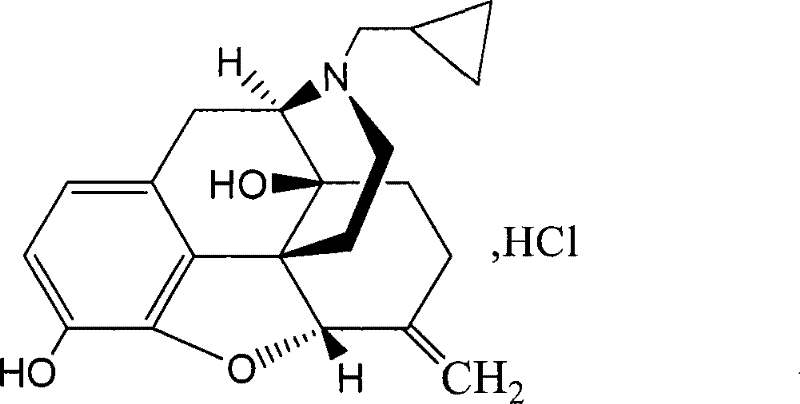
Nalmefene injection and preparation method thereof
A nalmefene and injection technology, which can be applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problem of stability of nalmefene hydrochloride injection, and cannot fully meet the quality requirements. Stability and other issues, to achieve the effect of good stability, reasonable formulation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] prescription:
[0035] Nalmefene Hydrochloride 0.1g
[0036] Sodium chloride 9g
[0037] Tryptophan 0.1g
[0038] Proper amount of hydrochloric acid to pH 3.8
[0039] Sterile water for injection to 1000ml.
[0040]Preparation process: 1) Take nalmefene hydrochloride and add 60% of the total amount of water for injection, and stir until completely dissolved; 2) Add the above-mentioned amount of sodium chloride and tryptophan, stir to dissolve, and add 30% of the total amount of water for injection; 3) ) Use 1.0mol / L hydrochloric acid solution to adjust the pH, and then add the remaining 10% water for injection; 4) Add 0.05% (w / v) activated carbon activated at 115-120°C for 2 hours, and keep stirring at 80°C for 30 minutes , coarsely filtered, decarbonized, and filtered with a composite filter membrane composed of 0.45 μm and 0.22 μm until clear; 5) Filled in a glass ampoule under nitrogen-filled conditions (fill 1ml of liquid in a glass ampoule with a volume of 1ml)...
Embodiment 2
[0043] prescription:
[0044] Nalmefene Hydrochloride 0.1g
[0045] Sodium chloride 9g
[0046] Tryptophan 0.05g
[0047] Proper amount of hydrochloric acid to pH 3.8
[0048] Sterile water for injection to 1000ml.
[0049] Preparation and sterilization process: the same as in Example 1. 1ml glass ampoules are used for dispensing, and the dispensing volume is 1ml. PH value after sterilization: measuring method is the same as embodiment 1, and the result is 3.9.
Embodiment 3
[0051] prescription:
[0052] Nalmefene Hydrochloride 0.1g
[0053] Sodium chloride 9g
[0054] Tryptophan 0.025g
[0055] Proper amount of hydrochloric acid to pH 3.8
[0056] Sterile water for injection to 1000ml.
[0057] Preparation and sterilization process: the same as in Example 1. PH value after sterilization: measuring method is the same as embodiment 1, and the result is 3.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com