Stable naloxone hydrochloride lyophilized preparation and preparation method thereof
A technology of naloxone hydrochloride and freeze-dried preparations, which can be used in freeze-dried delivery, nervous system diseases, powder delivery, etc. It can solve the problems of affecting patient safety, many excipients, unknown side effects, etc., and achieves good resolubility, few side effects, Recipe Simple Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
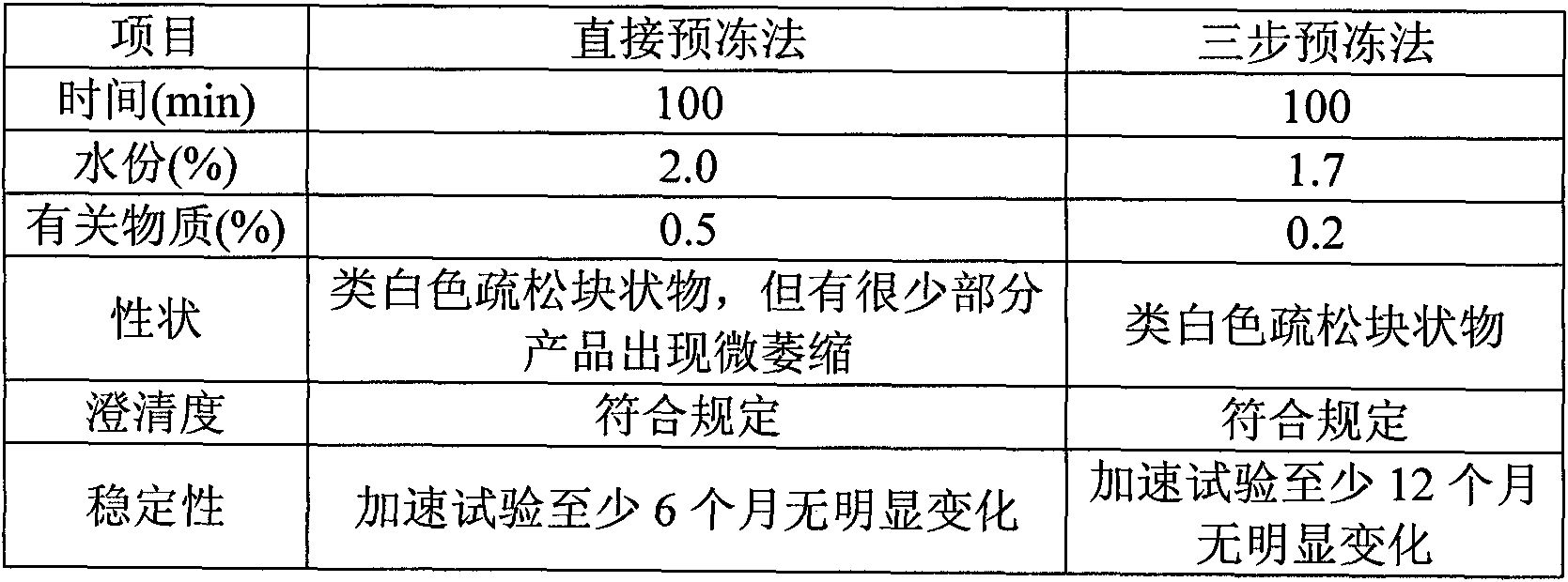
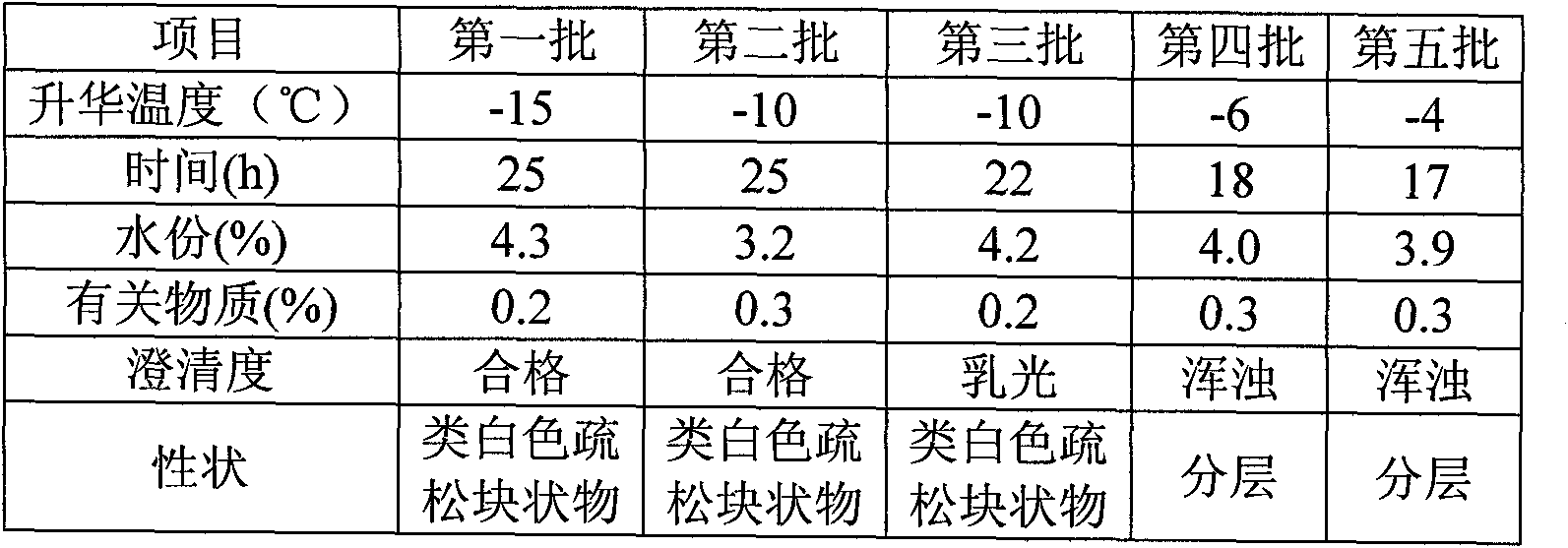
[0050] Naloxone hydrochloride 0.4g, mannitol 80g, mix naloxone hydrochloride and mannitol, add water for injection and stir until completely dissolved, adjust pH to 3.5 with hydrochloric acid and sodium hydroxide, add water for injection to 1000ml, add a total of 0.05 % of needles with activated carbon, stirred for 15 minutes, filtered and decarbonized, and the medicinal solution was finely filtered with a 0.22 μm sterile microporous filter membrane, and the pH value, content, and half stopper were measured, and then the medicinal product was put into a freeze-drying box for pre-freezing. -40°C for 2 hours. After the drug is frozen, start the vacuum machine to evacuate to 10Pa, turn off the freezer, and heat up the drug to keep the temperature of the frozen product at -40°C~-10°C; the time is 25h. Gradually raise the temperature to 25°C, keep warm and vacuum dry for 3 hours. A freeze-dried formulation was obtained.
Embodiment 2
[0052] Naloxone hydrochloride 2g, mannitol 60g, mix naloxone hydrochloride and mannitol, add water for injection and stir until completely dissolved, adjust pH to 4 with hydrochloric acid and sodium hydroxide, add water for injection to 1000ml, add a total of 0.05 % of needles with activated carbon, stirred for 15 minutes, filtered and decarbonized, and the medicinal solution was finely filtered with a 0.22 μm sterile microporous filter membrane, and the pH value, content, and half stopper were measured, and then the medicinal product was put into a freeze-drying box for pre-freezing. -40°C for 2 hours. After the drug is frozen, start the vacuum machine to evacuate to 10Pa, turn off the freezer, and heat up the drug to keep the temperature of the frozen product at -40°C~-10°C; the time is 25h. Gradually raise the temperature to 25°C, keep warm and vacuum dry for 3 hours. A freeze-dried formulation was obtained.
Embodiment 3
[0054] Naloxone hydrochloride 0.4g, mannitol 70g, mix naloxone hydrochloride and mannitol, add water for injection and stir until completely dissolved, adjust pH to 3 with hydrochloric acid and sodium hydroxide, add water for injection to 1000ml, add the total amount 0.05% activated carbon for needles, stirred for 15 minutes, filtered and decarbonized, finely filtered the drug solution with a 0.22 μm sterile microporous filter membrane, measured pH value, content, and half stoppered, and then put the drug into a freeze-drying box for pre-freezing. The temperature is -40°C, and the time is 2 hours. After the medicine is frozen, start the vacuum machine to evacuate to 10 Pa, turn off the freezer, and heat up the medicine to keep the temperature of the frozen product at -40°C ~ -10°C; the time is 25h. The medicine is gradually heated to 25°C, and vacuum-dried for 3 hours. A freeze-dried formulation was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com