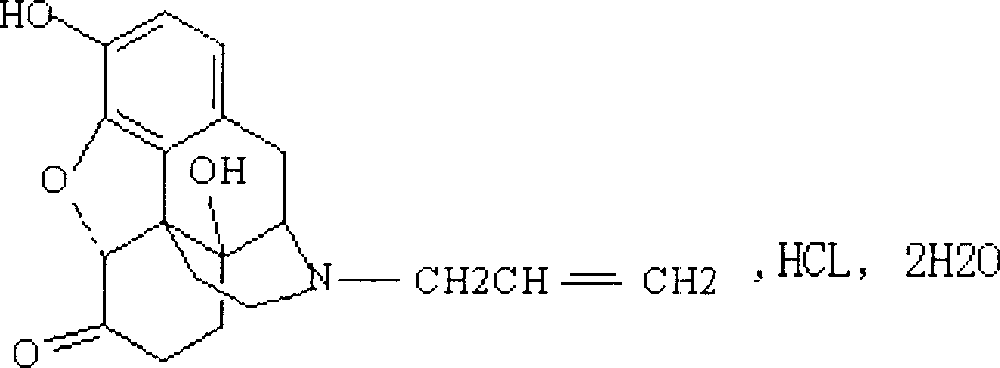Nasal cavity taken drug system and combination of naloxone hydrochloride and preparation method
A technology for nasal administration of naloxone hydrochloride, which is applied in drug combinations, pharmaceutical formulations, respiratory diseases, etc., can solve the problems of affecting bioavailability, large first-pass effect of the liver, and poor user compliance, and achieve rapid absorption , controllable quality and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Naloxone hydrochloride spray
[0024] Ingredient Amount
[0025] Naloxone Hydrochloride 80mg
[0026] Polyvinylpyrrolidone 0.5g
[0027] Laurazone 0.5ml
[0028] Propylene glycol 1.0g
[0029] Ethylparaben 0.1g
[0030] Distilled water to 100ml
[0031] Preparation method: Stir the above-mentioned amount of polyvinylpyrrolidone, naloxone hydrochloride, propylene glycol, laurozone and ethylparaben thoroughly to dissolve them all, and finally add distilled water to 100ml. The obtained solution is distributed in a spray pump or a quantitative drop pump.
Embodiment 2
[0032] Embodiment 2 Naloxone hydrochloride spray
[0033] Naloxone Hydrochloride 80mg
[0034] Hydroxypropyl beta-cyclodextrin 2.5g
[0035] Ethylparaben 0.1g
[0036] Mannitol 0.5g
[0037] Macrogol 400 1.0ml
[0038] Distilled water to 100ml
[0039] Preparation method: Shake the above amount of naloxone hydrochloride, hydroxypropyl β-cyclodextrin, ethylparaben, mannitol and distilled water to dissolve, then add the above polyethylene glycol 400, and finally add distilled water to 100ml .
Embodiment 3
[0040] Embodiment 3 Naloxone hydrochloride spray
[0041] Naloxone Hydrochloride 160mg
[0042]Methyl beta-cyclodextrin 5g
[0043] Ethylparaben 0.1g
[0044] Polyvinyl alcohol 0.5g
[0045] Mannitol 2-4g
[0046] Distilled water to 100ml
[0047] Steaming method: add distilled water to the above amount of naloxone hydrochloride, methyl β-cyclodextrin, ethylparaben, polyvinyl alcohol, shake to dissolve all, and finally add distilled water to 100ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com