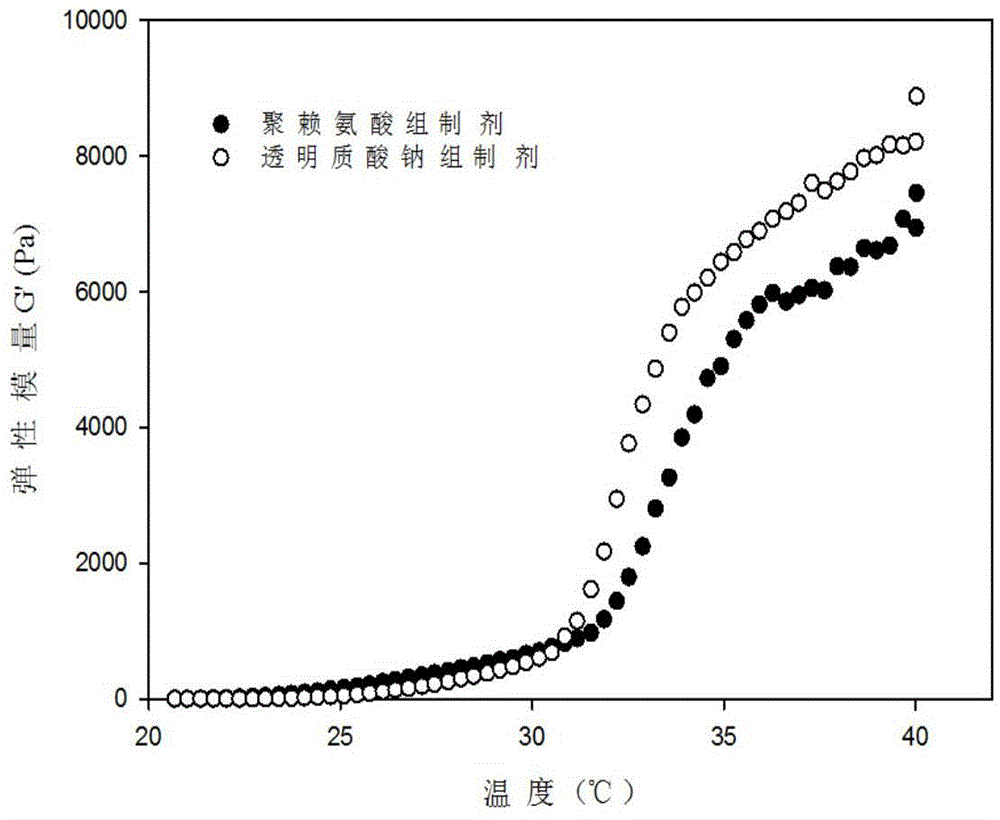
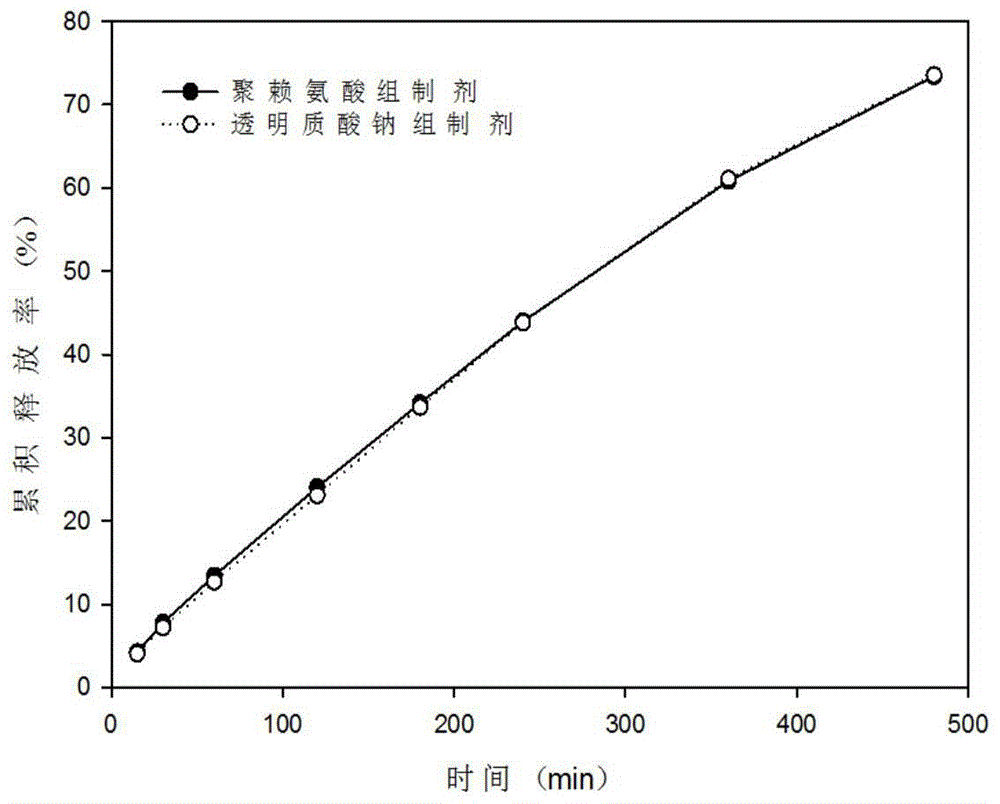
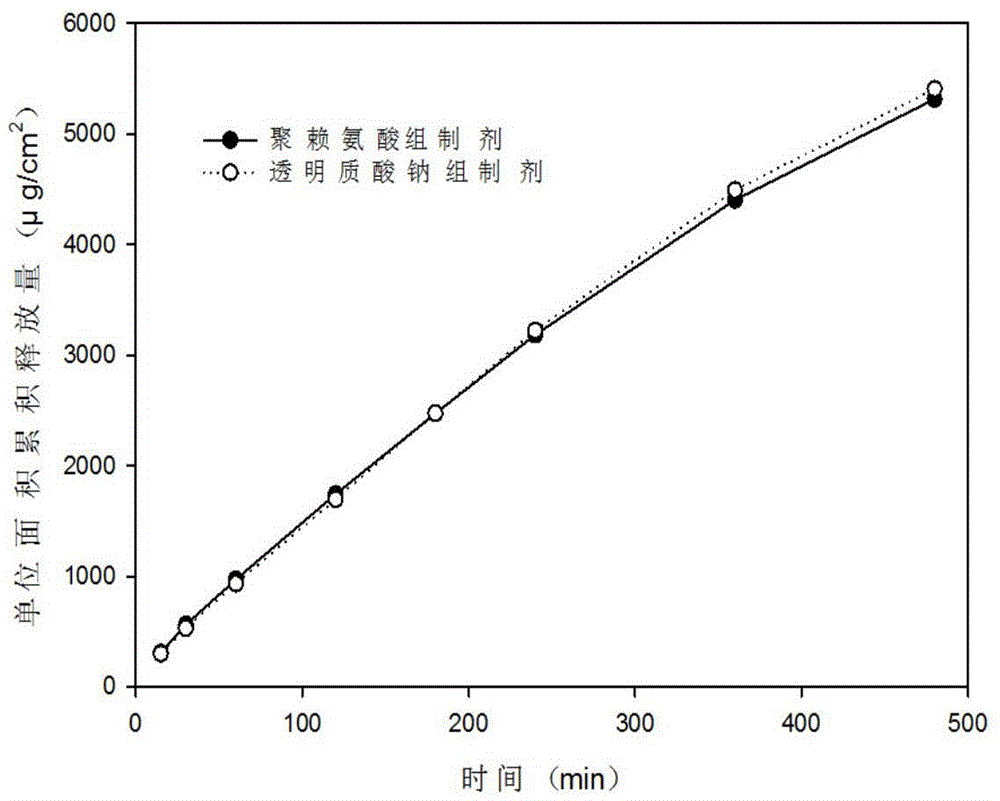
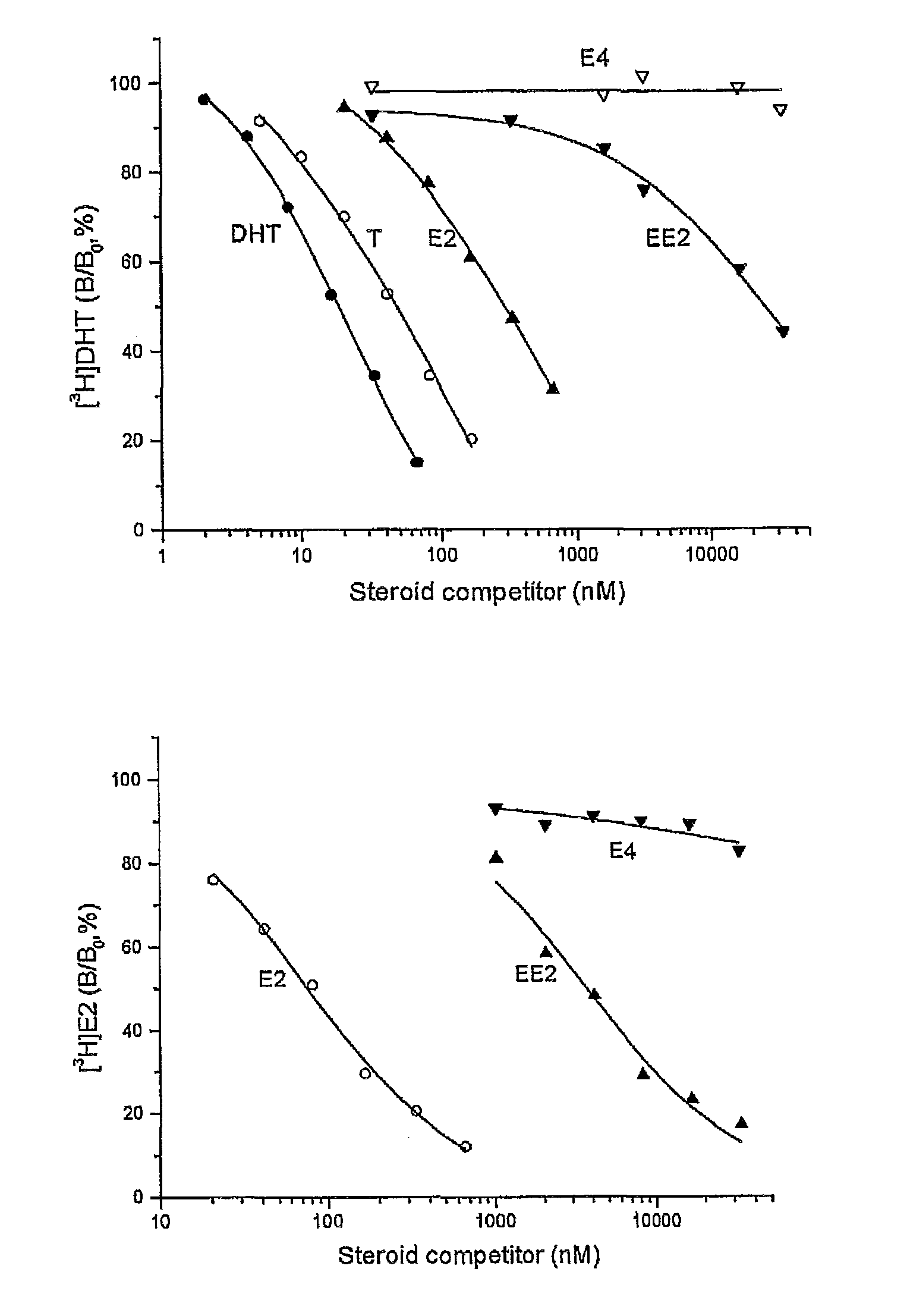
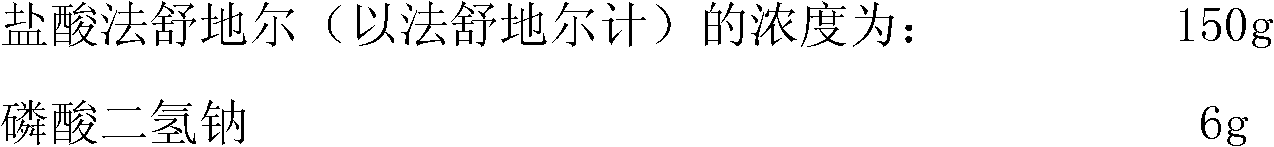Patents
Literature
37 results about "Metered Dose Nasal Spray" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
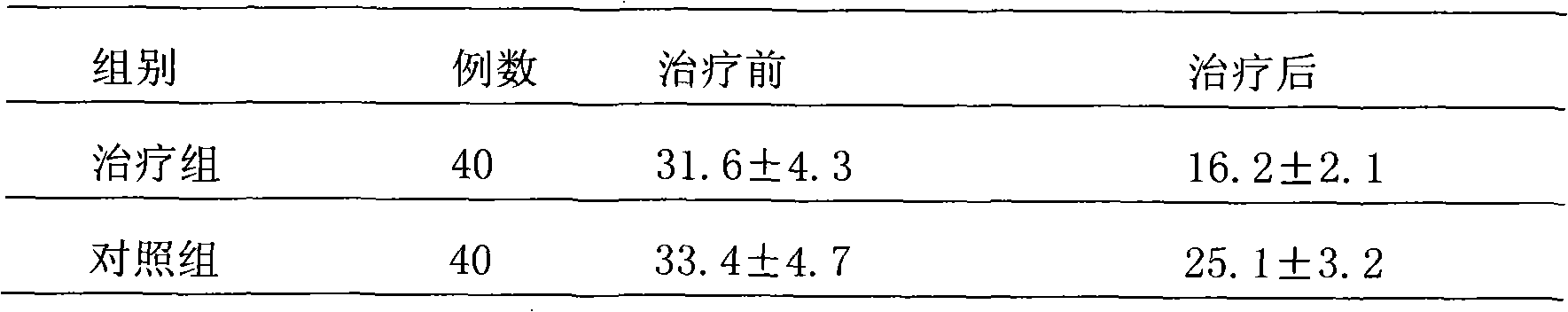
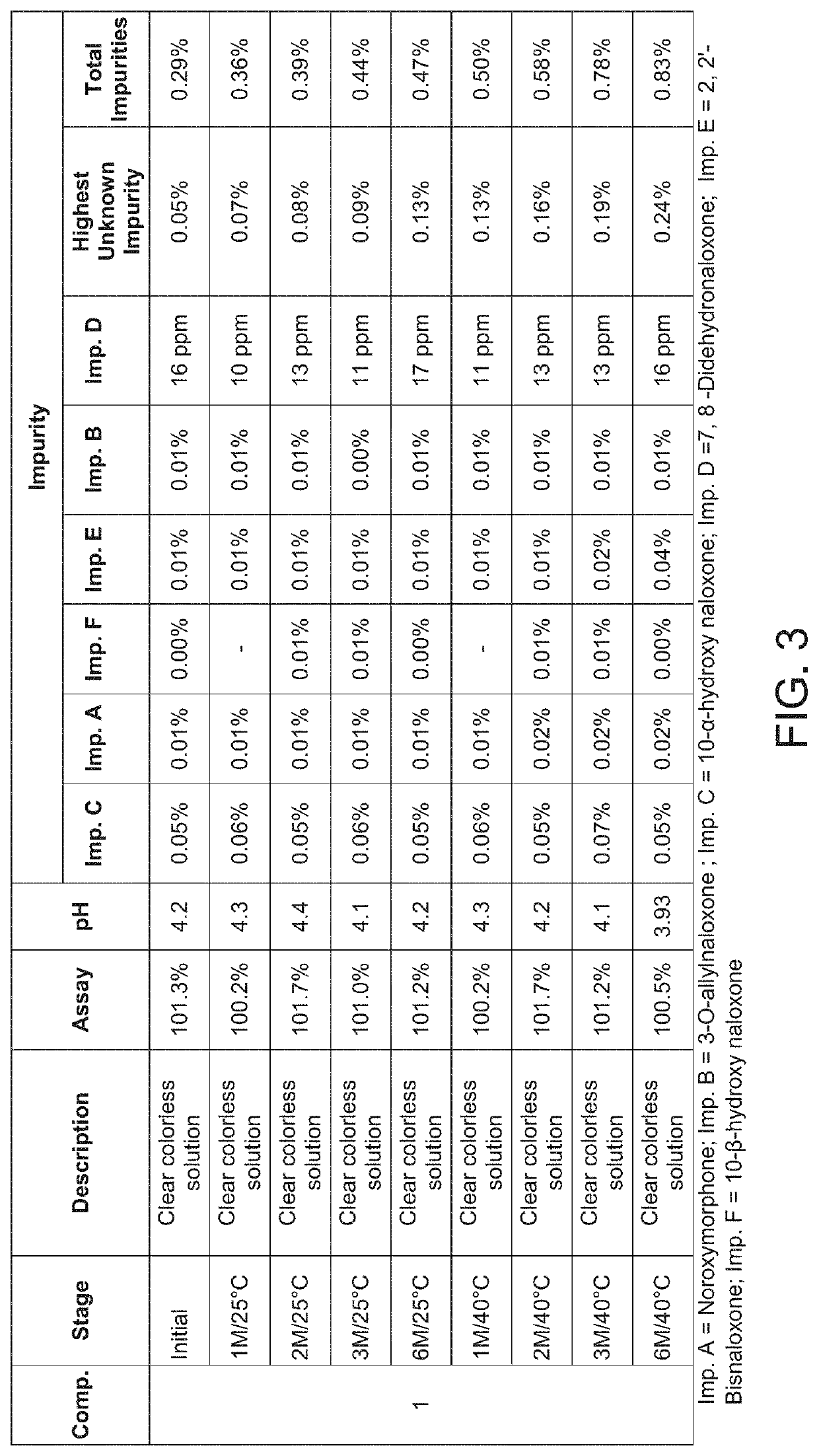
Naloxone hydrochloride nasal spray
The present invention is nasal cavity spray of Naloxine hydrochloride and its preparation process. The preparation of the present invention may be applied in single dosage form or multiple dosage form. The spray of the present invention includes Naloxine hydrochloride, osmotic pressure regulator, preservative, osmotic promoter and water.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Pharmaceutical composition comprising propofol
The invention provides novel pharmaceutical compositions comprising the active ingredient propofol. Preferably, propofol is dissolved in at least one semifluorinated alkane. The compositions, which are preferably liquid or gel-like, may optionally comprise further excipients. They may be used as fill material in capsules, as buccal or nasal sprays, or as aerosols for pulmonary administration. They are particularly useful for the transmucosal administration of propofol.
Owner:NOVALIQ GMBH
Pharmaceutical composition comprising propofol
The invention provides novel pharmaceutical compositions comprising the active ingredient propofol. Preferably, propofol is dissolved in at least one semifluorinated alkane. The compositions, which are preferably liquid or gel-like, may optionally comprise further excipients. They may be used as fill material in capsules, as buccal or nasal sprays, or as aerosols for pulmonary administration. They are particularly useful for the transmucosal administration of propofol.
Owner:NOVALIQ GMBH
Nasal temperature-sensitive type hydrogel spray
ActiveCN104546716AReverse thermosensitivityGuaranteed SprayabilityAerosol deliveryOintment deliveryNasal cavityGel preparation
The invention belongs to the field of medicinal preparations and relates to a nose temperature-sensitive type hydrogel spray which is prepared by dissolving a nasal decongestant, an anti-allergic rhinitis drug, a temperature-sensitive type material of poloxamer and a low viscosity bioadhesive material without mucosal toxicity into water. A gel preparation is prepared from gel temperature, the viscosity of the preparation in use is considered simultaneously, the temperature sensibility and sprayable performance of the preparation are ensured, then the preparation doses in a liquid spray manner at the room temperature (25 DEG C), the preparation is translated into semisolid gel with suitable intensity after being contacted with nasal mucosa surface, and the preparation is delayed on the nasal mucosa surface for a long time with the mucosa adhesion action of the bioadhesive. By utilizing the nose temperature-sensitive type hydrogel spray, patient medication is convenient. Compared with a common nasal cavity spray, by utilizing the nose temperature-sensitive type hydrogel spray, the residence time of the drug in a nose cavity can be prolonged so as to increase local absorption of the drug and prolong the pesticide effect.
Owner:CHINA PHARM UNIV
Nasal spray agent
The present invention is nasal spray of setron medicine includes the medicine component selected from Ondansetron, tropisetron and granisetron, osmotic pressure regulator and water.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Drug delivery system comprising a tetrahydroxylated estrogen for use in hormonal contraception
ActiveUS7871995B2Improve reliabilityReliable efficacyOrganic active ingredientsBiocidePresent methodPhysiology
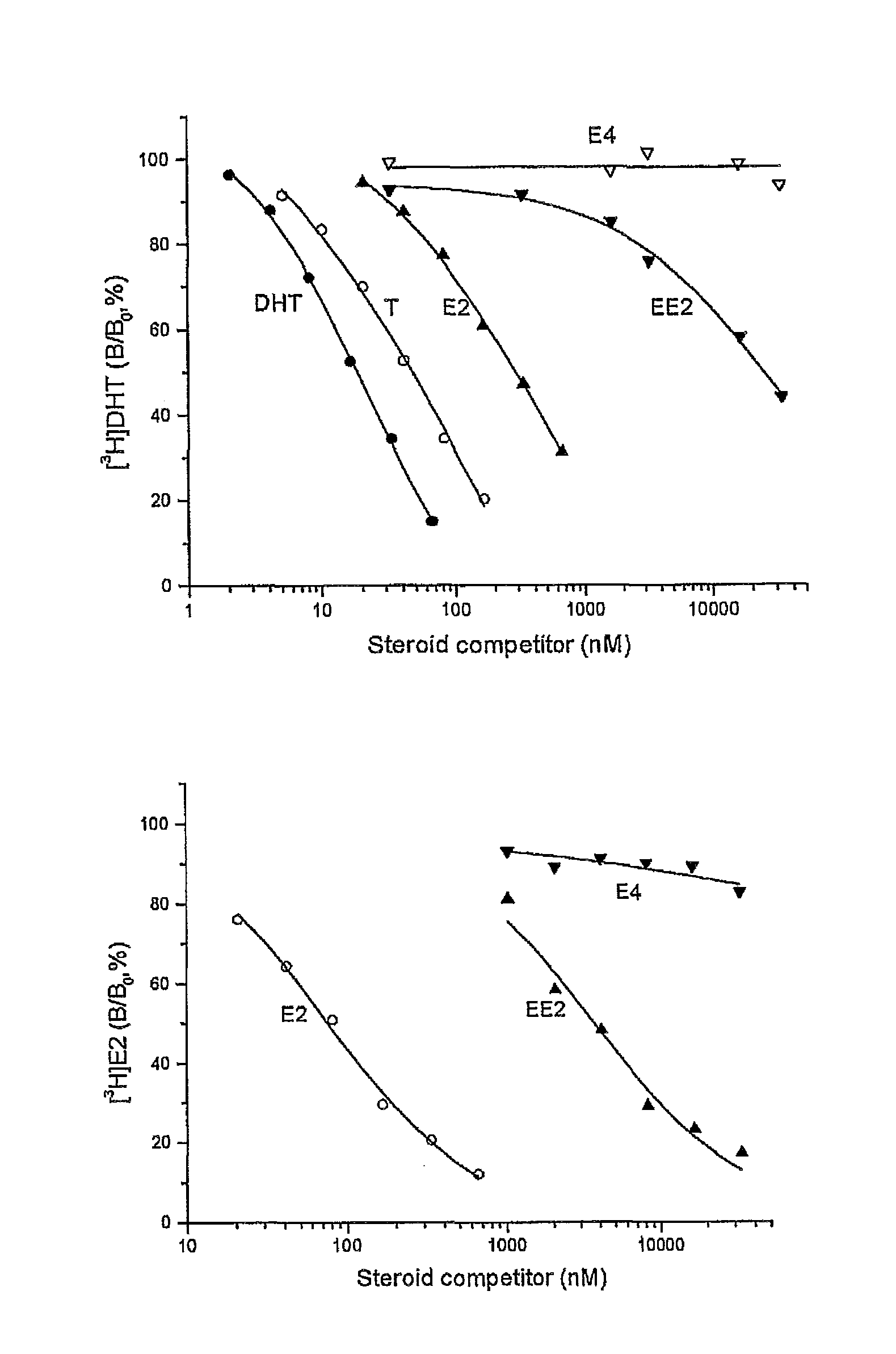
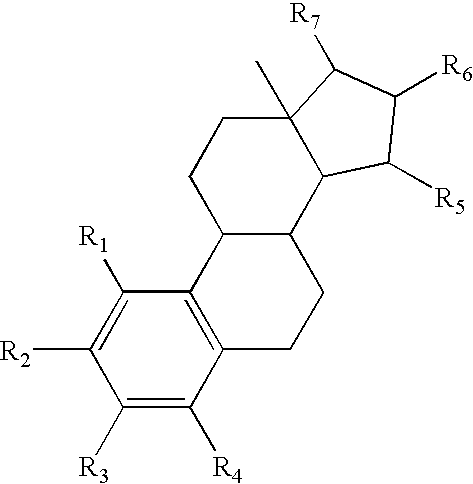
A method of contraception in mammalian females, which method comprises the parenteral or rectal administration of an estrogenic component and a progestogenic component to a female of childbearing capability in an amount effective to inhibit ovulation, wherein the estrogenic component is selected from the group consisting of substances represented by the following formula (1)in which R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alkoxy group with 1-5 carbon atoms; each of R5, R6, R7 is a hydroxyl group; and no more than 3 of R1, R2, R3, R4 are hydrogen atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method; and mixtures of one or more of the aforementioned substances and / or precursors. Another aspect of the invention concerns a drug delivery system for parenteral or rectal administration that contains the aforementioned estrogenic component and a progestogenic component, said drug delivery system being selected from the group consisting of suppositories, systems for intravaginal delivery, inhalers, nasal sprays and transdermal delivery systems.
Owner:ESTETRA SRL
Method for treating allergic rhinitis without adverse effects
The present invention is directed to a method for treating allergic rhinitis without causing an adverse effect of bitter taste. The method comprises administering to a patient an aqueous pharmaceutical formulation comprising 0.1-0.15% (w / v) of epinastine or an acid addition salt thereof, 0.05-0.5% (w / v) of hydroxypropylmethylcellulose to maintain the viscosity between 1.5-10 centipoise, 1-2% (w / v) of propylene glycol, and a buffer to maintain the pH between 5-8, said aqueous epinastine formulation has a tonicity between 200-400 mOsm / kG; the formulation does not contain a sweetening agent. The present invention provides a method for effectively treating allergic rhinitis by delivering a small volume of the epinastine formulation to the nose of a patient using a small volume metered-dose nasal spray pump. The present method does not cause an adverse effect of bitter taste without including sweetening agents in the formulation.
Owner:INSPIRE PHARMA
Nasal spray and preparation method thereof
InactiveCN109394837AWet long termLittle side effectsAntibacterial agentsHydroxy compound active ingredientsSide effectSea salt
The invention provides a nasal spray and a preparation method thereof. The nasal spray is prepared from sea salt, glycerin, sodium hyaluronate, ethylparaben, ethanol, potassium sorbate, a eucalyptus globulus leaf extracting solution, a bay leaf extracting solution, a sandalwood extracting solution and purified water through special processes and flows. The types of sterilizing agents in the nasalspray are changed, and natural sterilizing and inflammation-diminishing substances in plants of eucalyptus globulus leaves, bay leaves and sandalwood are adopted instead of the conventional antibioticmedicaments such as aspirin, ibuprofen and chlortrimeton, so that the side effects on human body are reduced, and bacteria in the nasal cavity are prevented from developing drug tolerance. The glycerin and the sodium hyaluronate are adopted for forming a layer of moisturizing film on the inner wall of the nasal cavity, so that the durability of the product in nasal cavity cleaning and moisteningis improved on the premise of removing pathogenic bacteria in the nasal cavity, and the nasal cavity can keep clean and wet for a long time.
Owner:吉林省七维生物科技有限公司
Nose temperature-sensitive type hydrogel spray
InactiveCN103705463AReverse thermosensitivityGuaranteed SprayabilityAerosol deliveryPharmaceutical non-active ingredientsGel preparationNasal cavity
The invention belongs to the field of medicinal preparations and relates to a nose temperature-sensitive type hydrogel spray which is prepared by dissolving a nasal decongestant, an anti-allergic rhinitis drug, a temperature-sensitive type material of poloxamer and a low viscosity bioadhesive material without mucosal toxicity into water. A gel preparation is prepared from gel temperature, the viscosity of the preparation in use is considered simultaneously, the temperature sensibility and sprayable performance of the preparation are ensured, then the preparation doses in a liquid spray manner at the room temperature (25 DEG C), the preparation is translated into semisolid gel with suitable intensity after being contacted with nasal mucosa surface, and the preparation is delayed on the nasal mucosa surface for a long time with the mucosa adhesion action of the bioadhesive. By utilizing the nose temperature-sensitive type hydrogel spray, patient medication is convenient. Compared with a common nasal cavity spray, by utilizing the nose temperature-sensitive type hydrogel spray, the residence time of the drug in a nose cavity can be prolonged so as to increase local absorption of the drug and prolong the pesticide effect.
Owner:CHINA PHARM UNIV
Xingnaojing nasal spray and preparation method and application thereof
InactiveCN102526619AReduce incidenceGood healing effectNervous disorderHydroxy compound active ingredientsMedicinal herbsDisease
The invention provides a Xingnaojing nasal spray and a preparation method thereof. The Xingnaojing nasal spray is prepared by taking musk, radix curcumae, cape jasmine and borneol as raw materials, and compounding above traditional Chinese medicinal herbs according to self characteristics of the traditional Chinese medicinal herbs. Through implementation of the Xingnaojing nasal spray, the incidence of die of illness and invalidism can be reduced, the disease healing effect can be improved, the course of treatment can be shortened, the prognosis can be improved, the recurrence can be prevented, and the swallowing function can be improved; and the Xingnaojing nasal spray has a relatively good therapeutic prospect.
Owner:赵联华
Traditional Chinese medicine sharpening-mind and inducing-consciousness microemulsion nasal cavity spraying agent and preparation method thereof
InactiveCN101596300AAvoid first passRealize the purpose of rescueNervous disorderHydroxy compound active ingredientsNasal cavityYolk
The invention relates to a traditional Chinese medicine sharpening-mind and inducing-consciousness microemulsion nasal cavity spraying agent and a preparation method thereof. The traditional Chinese medicine sharpening-mind and inducing-consciousness microemulsion nasal cavity spraying agent comprises 10 to 20 parts of artificial musk, 2 to 8 parts of borneol, 30 to 40 parts of turmeric, 40 to 50 parts of gardenia, 10 to 20 parts of polyoxyethylene-9-laurel ether, 5 to 8 parts of sulfo-lauryl alcohol, 1 to 6 parts of yolk phospholipid, 1 to 4 parts of lecithin, 2 to 10 parts of bean phospholipid, 2 to 4 parts of vitamin E, 10 to 30 parts of refined soybean oil, 10 to 20 parts of 1,2-propylene glycol, 10 to 15 parts of glycerol, 2 to 4 parts of cholic acid, a right amount of ethanol, a right amount of purified water, a right amount of essence and a right amount of propellant. The traditional Chinese medicine sharpening-mind and inducing-consciousness microemulsion nasal cavity spraying agent has the functions of clearing away heat and toxic material, removing heat from the blood, cooling blood, promoting blood circulation, sharpening mind and inducing consciousness, is natural, innoxious, safe, effective and biodegradable, is clinically used for treating various cerebrovascular diseases and is especially effective to central coma diseases, such as disturbance of consciousness, serious symptom craniocerebral injury, cerebral hemorrhage, cerebral infarction, drug poisoning, and the like; and the medicine realizes cerebral targeting administration by the approach of a nose, can break through blood-brain barriers, can rapidly exert actions and also has convenient administration.
Owner:广东同德药业有限公司
Medicinal composition for treating impotence
InactiveCN1977846AOrganic active ingredientsPharmaceutical delivery mechanismTraditional medicineDosage form
Owner:刘宝顺
Naloxone hydrochloride spraying agent for mouth and nose
InactiveCN101036651AEasy to useQuick effectOrganic active ingredientsAerosol deliveryNasal cavitySide effect
The invention discloses a naloxone hydrochloride spraying agent for oral or nasal cavity, which is prepared by naloxone hydrochloride, naloxone free alkali or the other naloxone acceptable in medicine having a ratio by weight of 1:2.5~10 with pharmaceutic edjuvant based on general spraying agent preparation process. Active ingredient of the naloxone hydrochloride spraying agent for oral or nasal cavity in the invention can directly participate intracorporeal circulation after absorbed by capillary tube under oral or nasal mucosa of a patient, thus has advantages of fast absorption and high bioavailability, without stimulating to the oral or nasal mucosa. In particular, the drugs of the invention can be taken with no requirement of use situation, so that it is convenient for using which dosage is controllable and has little side effect.
Owner:薛京 +1
Nasal cavity spraying agent and preparation method thereof, and biological barrier film
ActiveCN106821980AEffective barrierImprove barrier propertiesHydroxy compound active ingredientsAerosol deliveryGlycerolSolvent
The invention belongs to the medical field, and in particular, relates to a nasal cavity spraying agent and a preparation method thereof, and a biological barrier film. The nasal spraying agent provided by the invention includes cellulose, a moisturizing agent and a solvent; the cellulose includes hydroxypropyl methyl cellulose with the type of E4M and / or hydroxyethyl methyl cellulose with the type of 20WR, and the content of the cellulose in the nasal cavity spraying agent is 9-13 g / L; the moisturizing agent includes one or more of glycerol, propylene glycol and sorbitol, and the content of the moisturizing agent in the nasal cavity spraying agent is 70-130 g / L. After the nasal cavity spraying agent is sprayed into a nasal cavity, the nasal cavity spraying agent can be evenly distributed in the nasal cavity and rapidly forms a film, and a formed film is uniform and has good barrier effect on most allergens. Experimental results show that the biological barrier film formed by the nasal cavity spraying agent provided by the invention can effectively block most allergens with the particle size of PM 2.5 or more.
Owner:成都博创必成生物技术有限公司
Pharmaceutical composition containing interferon and application
InactiveCN103341160AReduce dependenceEliminate side effectsPeptide/protein ingredientsAerosol deliveryPatient complianceInterferon alpha
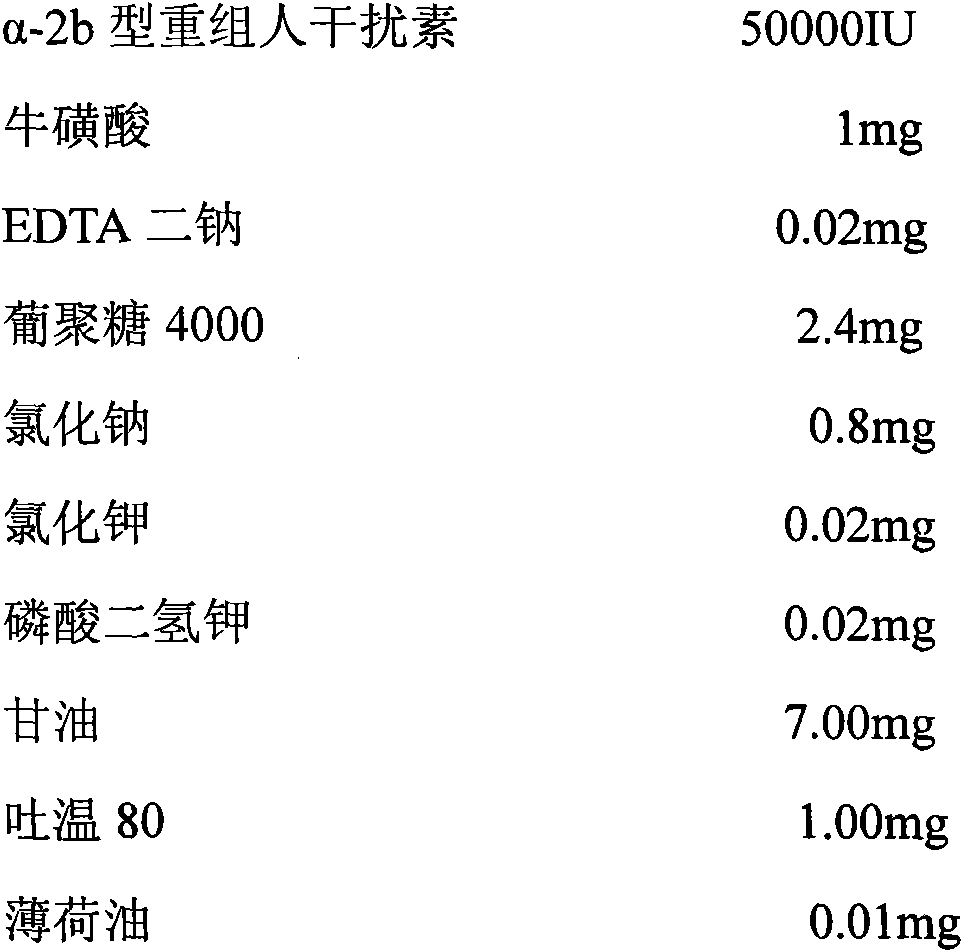
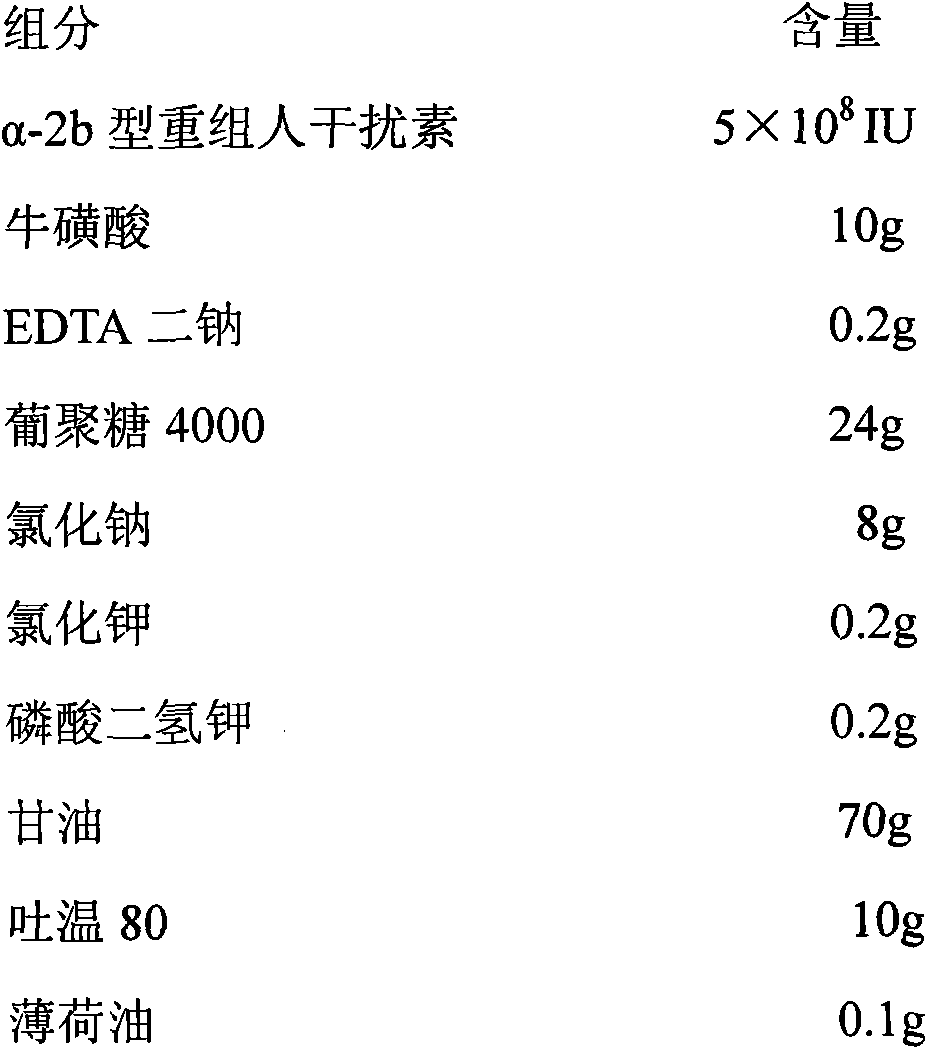
The invention discloses a pharmaceutical composition containing interferon and an application. Pharmacologically active ingredients of the pharmaceutical composition are the interferon and taurine, wherein an activity unit content of the interferon is 10 to 1x10<6> IU / mL, and a content of the taurine is 0.05-0.5 mg / ml. The pharmaceutical composition containing the interferon provided by the invention has effects of resisting activity of vesicular stomatitis virus and murine encephalomyocarditis virus, and protecting cells. Especially, each of nasal spray agent provided by the invention only has 50000 IU of alpha-2b recombinant human interferon and 1 mg of the taurine, but has a specific anti-viral activity of 9.15x10<5> to 2.8x10<6> IU, and shows substantial synergistic effect, thereby being significant for improving patient compliance and reducing medication costs, and meeting clinical needs of interferon medications.
Owner:澳蒲生物科技(上海)有限公司 +1
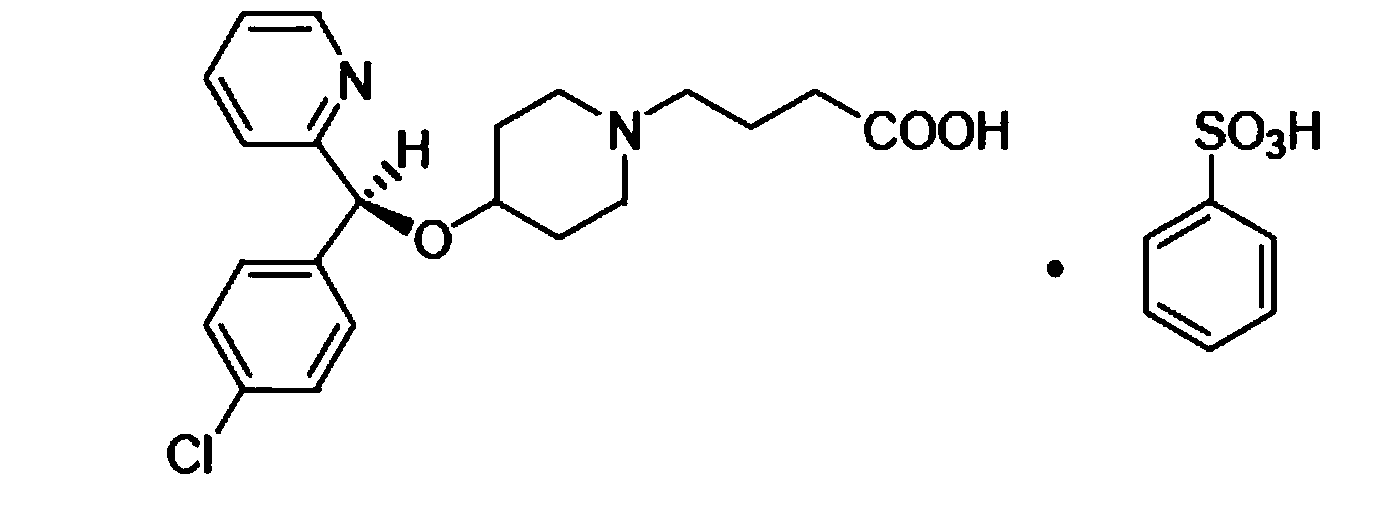
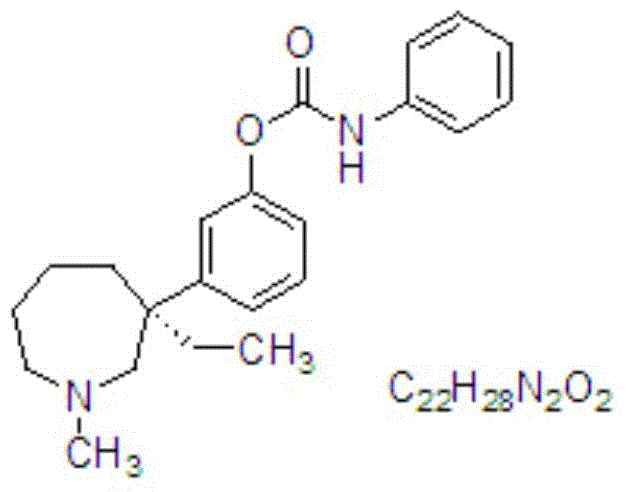
Bepotastine besilate nasal spray and preparation method thereof
ActiveCN103816121AOrganic active ingredientsAerosol deliveryMucous membrane inflammationPolyethylene glycol
The invention discloses a bepotastine besilate nasal spray and a preparation method thereof. For the nasal spray, 1-10 g of bepotastine besilate and 3-15 g of solubilizing composition are added in 100 mL of water; the solubilizing composition comprises components in percentage by weight as follows: 80%-95% of propylene glycol, polyethylene glycol 300 or polyethylene glycol 400 and the balance of caprylocaproyl macrogolglycerides; and pH of a nasal spray solution ranges from 6 to 8. In the composition, bepotastine besilate does not exist in a solid particle mode and cannot be crystallized during a long-term storage process, so that not only is rapid medicine absorption facilitated, but also a spray pump port is not blocked easily, and anaphylactic rhinitis and mucous membrane inflammation related to rhinitis can be cured effectively.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Dri nasal sprays
There are provided safe, tasteless, odorless, non-aqueous liquid spray compositions formulated for administration to the nasal cavity consisting essentially of pharmacologically acceptable non aqueous liquid carrier in which said bioactive material is directly insoluble, a pharmacologically acceptable water insoluble ester of a water soluble acid soluble in said carrier, a pharmacologically acceptable water soluble glycol soluble in said ester and a pharmacologically acceptable water soluble bio-active material soluble in said glycol but not directly soluble in the carrier. There are also provided methods of producing and administering such compositions.
Owner:MACKLES LEONARD
Nasal liquid medicine for treating headache and its prepn process
InactiveCN1973847AImprove absorption rateHigh yieldNervous disorderAerosol deliveryNasal InhalantNose
The present invention is one kind of nasal liquid medicine for treating headache and its preparation process. The medicine consists of volatile dahurian angelica oil and volatile Chuanxiong oil as the effective medicine components and pharmaceutically acceptable supplementary material. The medicine is prepared into emulsion form or gel form, such as nasal drop, nasal spray, aerosol, nasal inhalant, etc. The volatile oil as the effective component is preferably extracted through supercritical CO2 circular extraction and separation. The medicine for treating headache has high bioavailability and excellent effect.
Owner:成都厚发科技开发有限公司
Nasal spray
InactiveCN106309558ASignificant effectHigh cure rateHydroxy compound active ingredientsOrganic compound preparationCelluloseParanasal sinusitis
The invention provides a nasal spray which is composed of, by weight, 8-10 parts of small centipeda herb, 6-7 parts of flos magnolia, 12-15 parts of menthol, 34-51 parts of seawater, 5-7 parts of plant cellulose, 1-2 parts of isoosmotic adjusting agent, 1-2 parts of scutellaria baicalensis, 6-8 parts of sophora flavescens, 5-8 parts of mint and 5-7 parts of agastache rugosus. Compared with the prior art, the nasal spray has the advantages that the small centipeda herb can treat nasal obstruction and nasosinusitis, menthol is used as a stimulant pharmaceutically, acts on skin or mucosa and has effects of cooling and stopping itching, can treat acute and chronic rhinitis, allergic rhinitis, hypertrophic rhinitis, nasosinusitis and paranasal sinusitis, and other medicinal excipients are added, so that the nasal spray is protruding in treatment effect, high in healing rate, simple, convenient, cheap and easy to clinically popularize.
Owner:默比(广州)药业有限公司
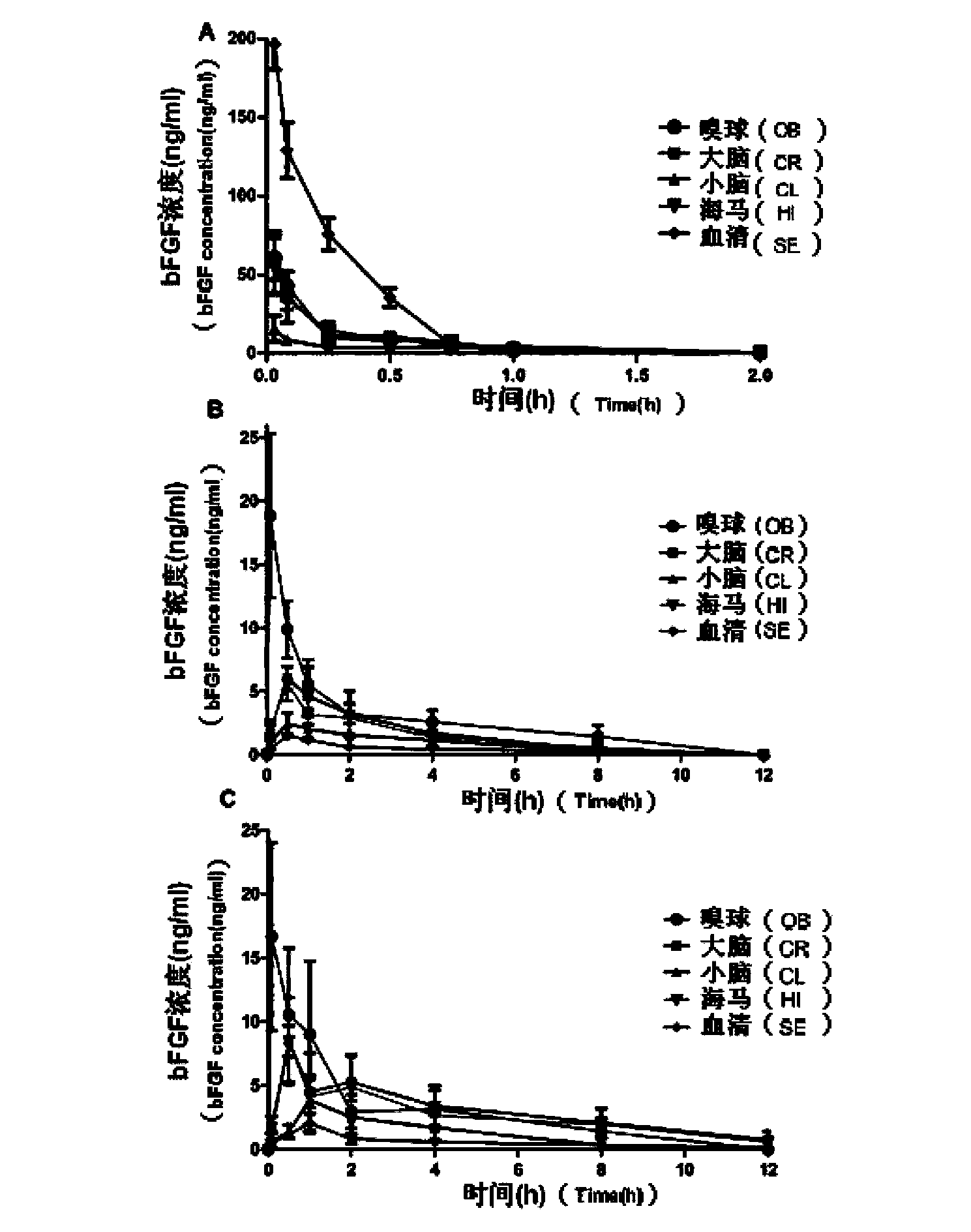
Basic fibroblast growth factor nasal spray for treating Alzheimer's disease
InactiveCN103768017AFix stability issuesSolve easy to be cleared by nasal ciliaNervous disorderPeptide/protein ingredientsNasal cavityDisease
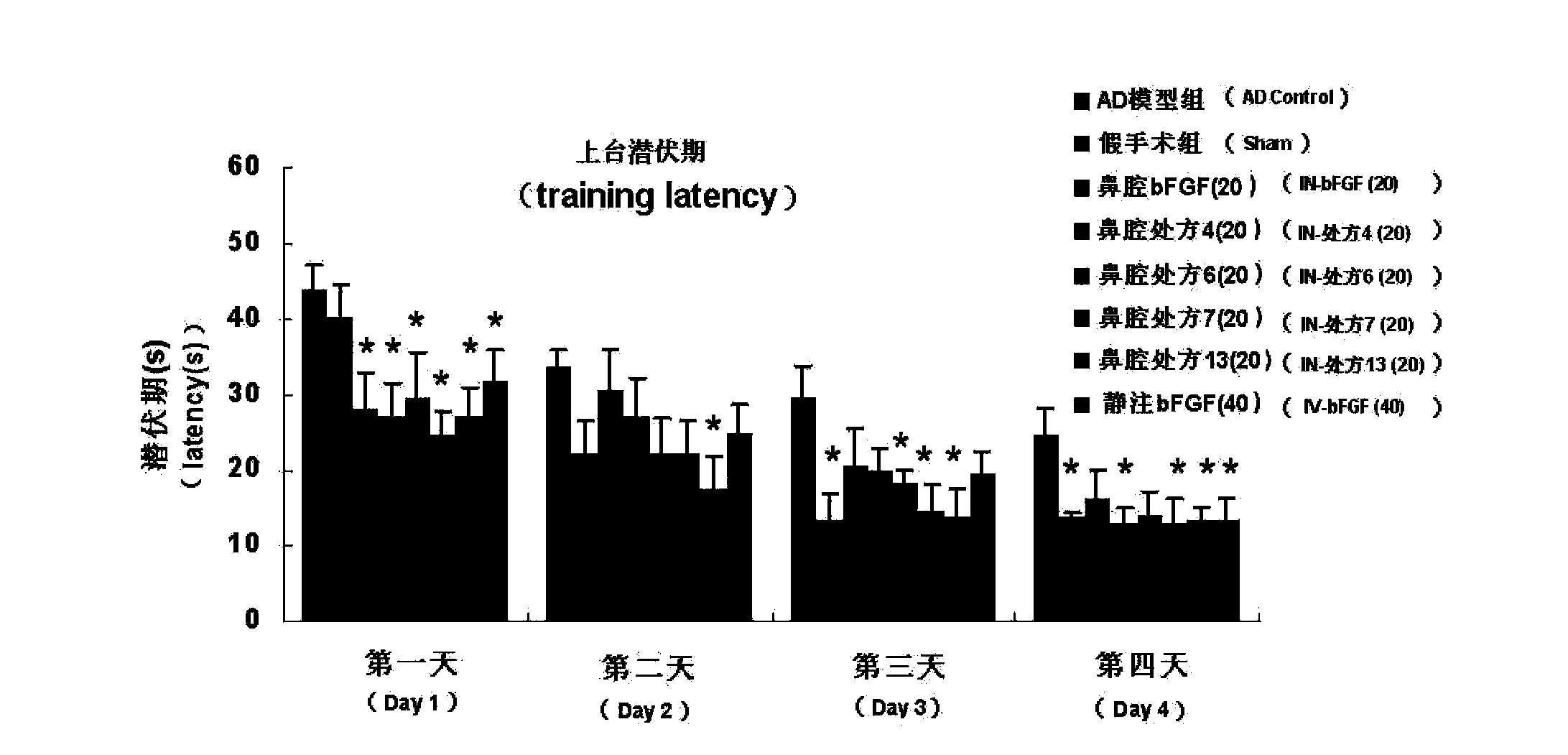
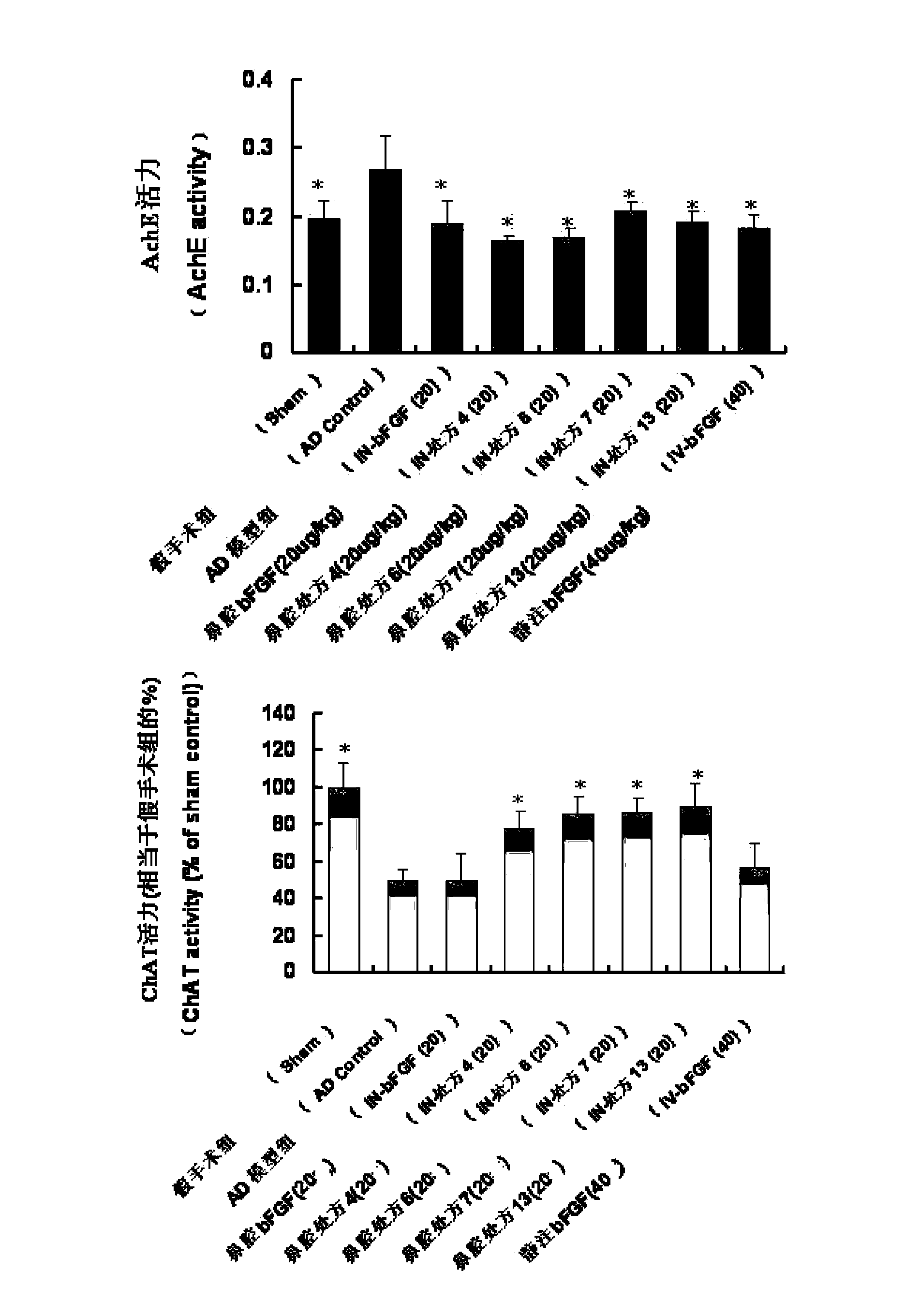
The invention belongs to the field of pharmaceutical manufacturing, and relates to a basic fibroblast growth factor nasal spray for treating the Alzheimer's disease. The nasal spray comprises basic fibroblast growth factors, sorbefacients, stabilizers, bioadhesion agents and other pharmaceutically effective auxiliary materials. Experiment results show that the nasal spray can remarkably increase the in-brain amount of the basic fibroblast growth factors, improve the treatment effect on the Alzheimer's disease, reduce the peripheral toxic or side effects of the drugs, and be used as the new basic fibroblast growth factor preparation for treating the Alzheimer's disease.
Owner:FUDAN UNIV
Nasal spray for treating headache and hemicrania and its preparation method
InactiveCN1579448AFast absorptionImprove bioavailabilityOrganic active ingredientsNervous disorderNasal cavityNose
The invention relates to a nose cavity spraying agent for curing headache and migraine and its manufacutirng method. The prescription includes medicine effective ingredients and accessories, the effective ingredients of the medicine is made up of 1-10 shares of chuanxiong rhizome extract, asarum extract and mint extract, the extraction of the effective ingredient may be steam distilling extract method or supercritical fluid extraction method, then the accessories are dissolved in the solution waterless alcohol, then adds in the effective ingredients of each medicine, they are blended fully and poured into the nose cavity spraying pump, the nose cavity spraying agent uses the rich mucous and vessels in the nose cavity, the absorption of the medicine is quick, the biology utilization rate is high, the effect is prominent, and it has no propellant, it does not use pressure resisting container, the atomization drop is tiny, it is even in the nose cavity.
Owner:ZHEJIANG UNIV OF TECH
Dri-nasal sprays
InactiveUS20040057907A1Aerosol deliveryPharmaceutical non-active ingredientsNasal cavityWater insoluble
There is provided non-aqueous liquid spray compositions comprising a pharmacologically acceptable non aqueous liquid carrier in which said bioactive material is directly insoluble, a pharmacologically acceptable water insoluble ester of a water soluble acid soluble in said carrier, a pharmacologically acceptable water soluble glycol soluble in said ester and a pharmacologically acceptable water soluble bio-active material soluble in said glycol but not directly soluble in the carrier. There are also provided methods of producing and administering such compositions.
Owner:MACKLES LEONARD
Traditional Chinese medicine preparation for treating sick headache
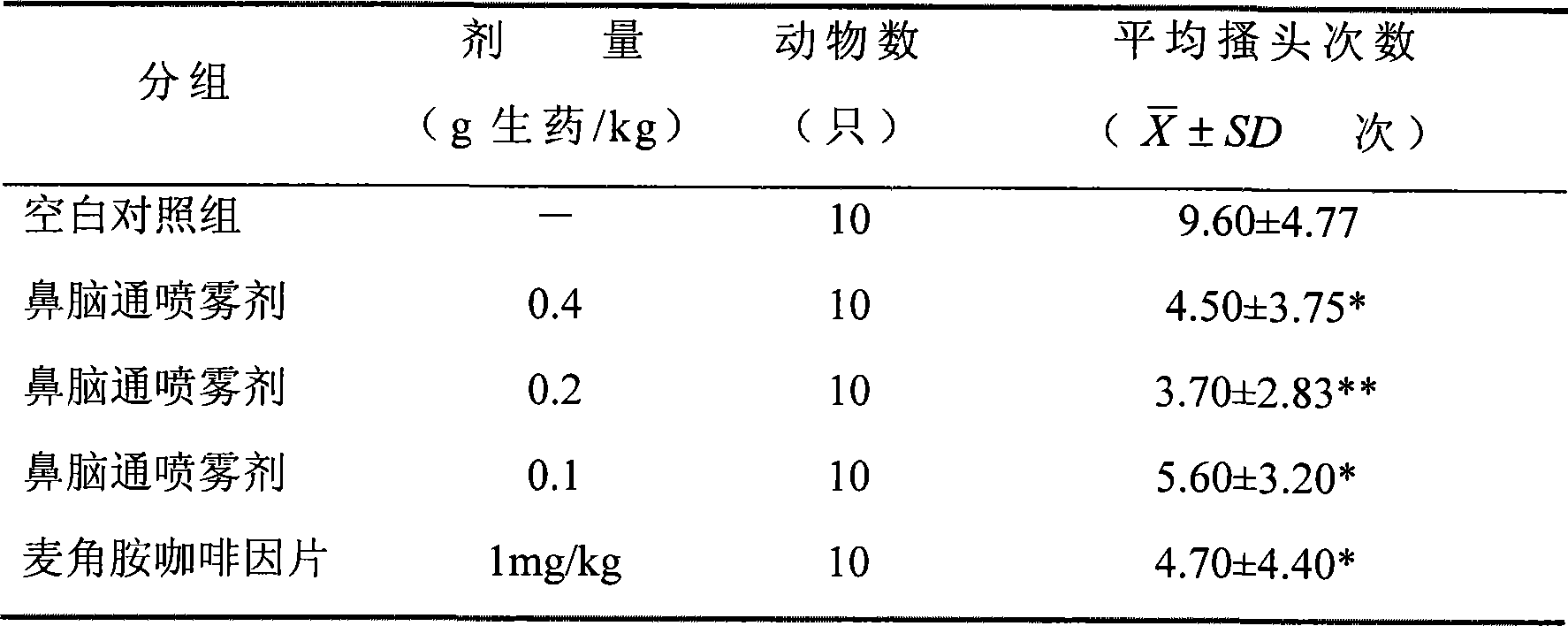
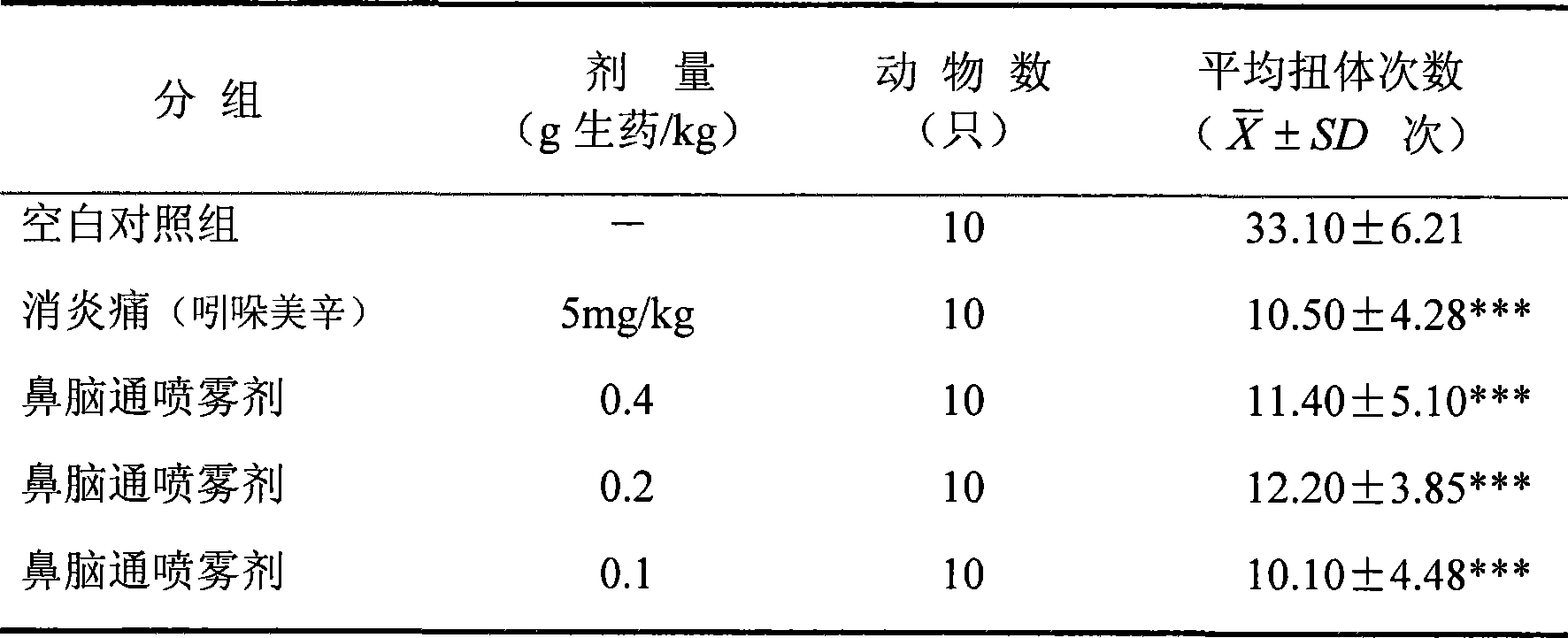
ActiveCN101219164AAchieve the purpose of treating migraineDiastolic adjustmentNervous disorderHydroxy compound active ingredientsNasal cavitySide effect
The invention discloses a traditional Chinese medicine preparation for curing migraine, which is prepared by extracting 1 proportion of rhizoma ligustici wallichi, 0.45-1.2 proportions of cinnamomum migao H.W.Li, 0.40-1.1 proportions of raphani, 0.005-0.15 proportion of artificial musk, 0.002-0.03 proportion of borneol and additives, in addition, nasal cavity spraying agents and nose drops can also be prepared. According to the traditional Chinese theory of 'the brain is linked with the nose', the medicine is taken through the nasal cavity, which has rapid pain relieving effect, high potency and no toxicity and side effects.
Owner:中国药材公司
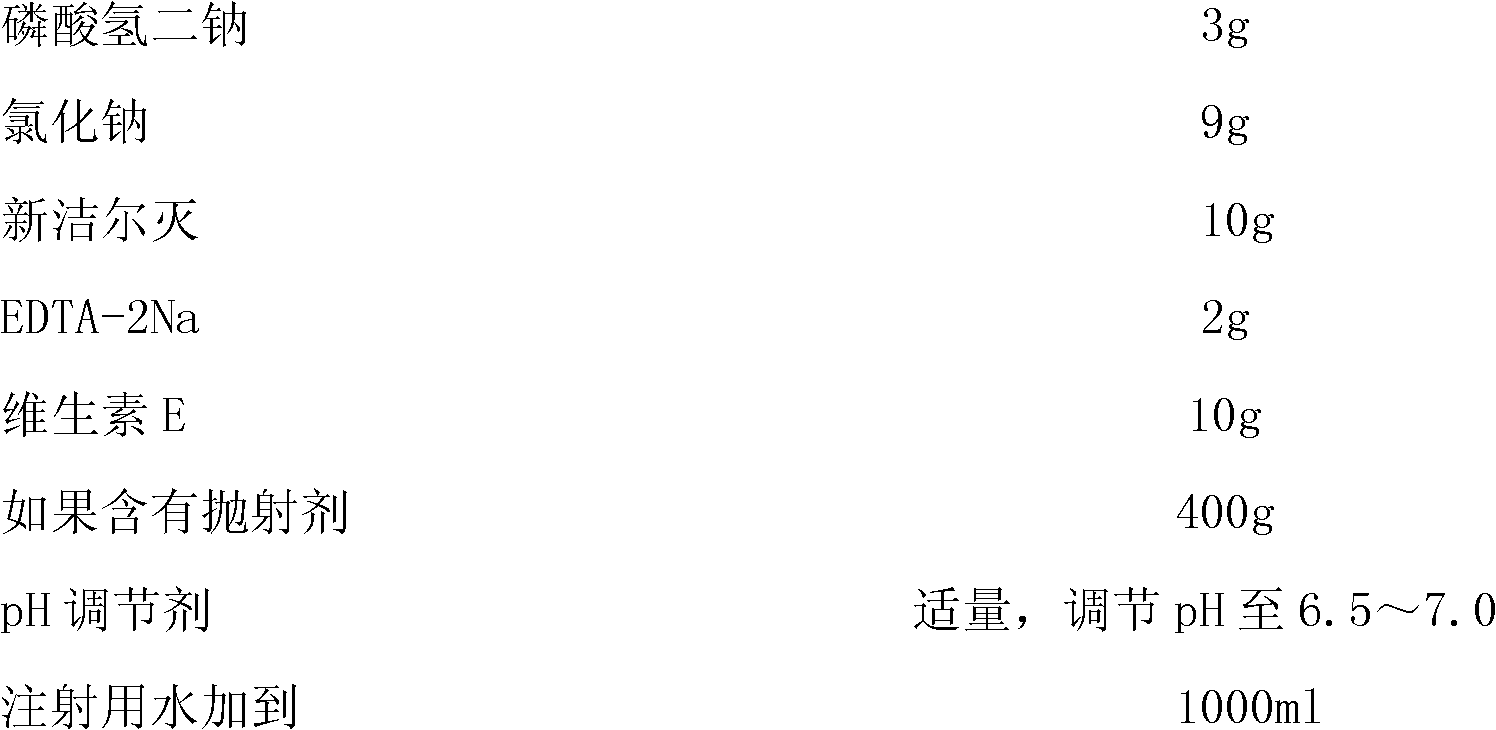
Nasal spray or aerosol containing Fasudil
ActiveCN102138901AImprove absorption rateShould not be toxicOrganic active ingredientsLiposomal deliveryActive componentNasal spray
The invention relates to a nasal spray or aerosol containing Fasudil. The active component in the preparation can be absorbed rapidly through the nasal mucosa so that the response is rapid. The nasal spray or aerosol has the advantages of high bioavailability and good compliance, and is convenient in use and carrying. The preparation provided by the invention contains Fasudil hydrochloride and pharmaceutically acceptable excipients.
Owner:TIANJIN CHASE SUN PHARM CO LTD
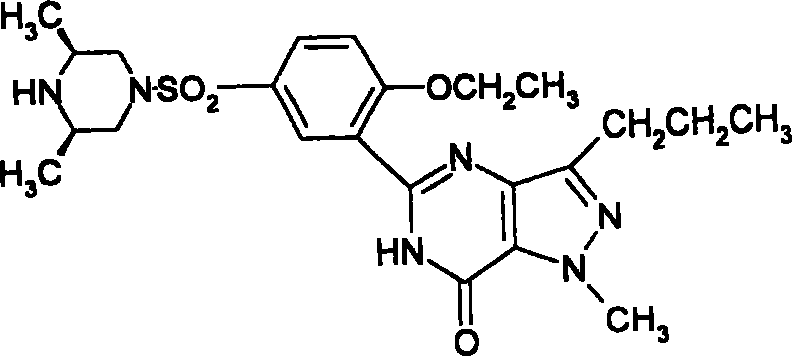
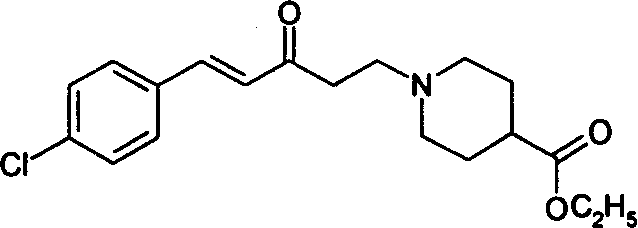
Compound for treating pulmonary fibrosis
The invention discloses the usage of the compound in a structural formula I in preparing drugs preventing and / or treating lung fibrosis of mammalian. The drugs can be made into orally-taken administration formulation selected from tablet, capsule, powder, granule, crystal, solution, suspension, decoction, syrup, elixirs, tea and masticatory spasm and also can be made into inhale administration or insufflation administration, intranasal administration and transdermal administration or administration formulation selected from spray of nasal cavity, nasal drop, suspension, gel, ointment, cream, emulsion and powder.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
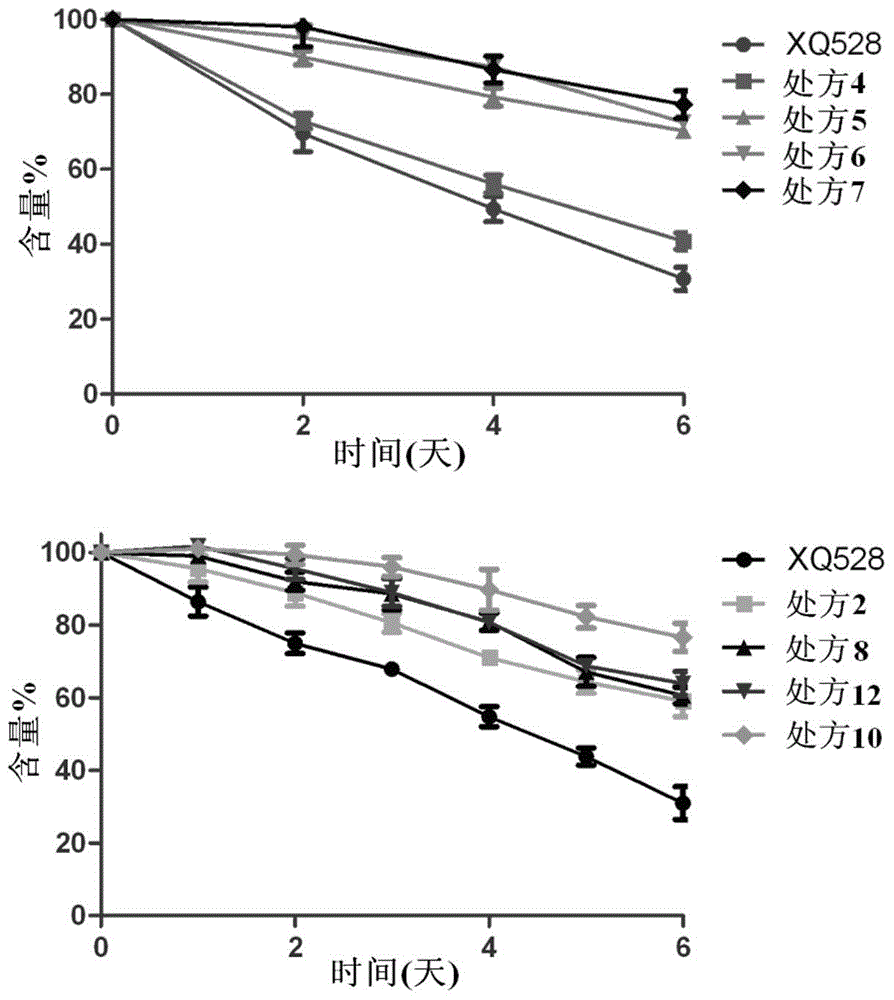
Nasal spray with cholinesterase inhibitors XQ528
InactiveCN105310982AAlleviation of pathological featuresPromote absorptionNervous disorderAerosol deliveryDiseaseNasal cavity
The invention relates to the field of medicine preparations, in particular to novel nasal spray with cholinesterase inhibitors XQ528. The novel nasal spray which is a preparation comprises the cholinesterase inhibitors XQ528, stabilizers and other pharmaceutically effective excipients, can be in an aqueous solution form or an in-situ gel form and can be administered in a spray mode. The novel nasal spray has the advantages that the stability of the cholinesterase inhibitors XQ528 can be improved, hydrolysis of the cholinesterase inhibitors XQ528 can be reduced, the nasal retention time of medicines can be prolonged, and nasal absorption of the medicines can be promoted; the novel nasal spray is mainly used for treating Alzheimer's diseases, improving memory impairment of patients suffering from the Alzheimer's diseases and relieving pathogenic symptoms.
Owner:FUDAN UNIV
Nasal liquid medicine for treating headache and its preparation process
InactiveCN100536860CImprove absorption rateHigh yieldNervous disorderAerosol deliveryEmulsionNasal Inhalant
The present invention is one kind of nasal liquid medicine for treating headache and its preparation process. The medicine consists of volatile dahurian angelica oil and volatile Chuanxiong oil as the effective medicine components and pharmaceutically acceptable supplementary material. The medicine is prepared into emulsion form or gel form, such as nasal drop, nasal spray, aerosol, nasal inhalant, etc. The volatile oil as the effective component is preferably extracted through supercritical CO2 circular extraction and separation. The medicine for treating headache has high bioavailability and excellent effect.
Owner:成都厚发科技开发有限公司
Nasal spray for preventing flu based on red pepper extract and heparin and preparation method thereof
InactiveCN112472669AAntibacterial and anti-inflammatoryLong moisturizing timeAntibacterial agentsOrganic active ingredientsBiotechnologyNasal cavity
The invention belongs to the technical field of medicine preparation, and particularly relates to a nasal spray for preventing flu based on a red pepper extract and heparin and a preparation method thereof. The nasal spray is prepared from the following raw materials in parts by weight: 10% of gamma-PGA-CS, 5-10% of glycerol, 2% of a red pepper extract, 5-10% of a heparin solution, 2-2.4% of ethanol and the balance of purified water. The nasal spray can inhibit bacteria and diminish inflammation, has a good treatment effect on rhinitis, can keep the inner surface of the nasal cavity moist fora long time, and reduces side effects on a human body by changing the types of bactericides in the nasal spray, so that the nasal cavity is kept clean and moist for a long time.
Owner:QUFU NORMAL UNIV
Multiple Dose Nasal Spray of Naloxone
A multiple dose Naloxone spray dosage form provides a container having two or more unit doses of Naloxone or a salt thereof in a liquid that is delivered by a metered dosage spray pump. In different embodiments, ten or more, 3-500, and 10-500 unit doses are provided in the container. The metered dosage spray pump delivers 50 to about 200 μL of Naloxone solution per spray, preferably, 100 μL per spray. Each unit dose contains about 0.5 mg to 12 mg of Naloxone or a pharmaceutically acceptable salt thereof, preferably, about 2 mg to 4 mg thereof in the delivered spray. A stable pharmaceutically acceptable composition of Naloxone is used in the multiple dose Naloxone spray dosage form. A method of treatment of opioid overdose involves administration of multiple doses using the multiple dose Naloxone spray dosage form.
Owner:NAVINTA III INC
A kind of traditional Chinese medicine nasal cavity anti-inflammatory preparation and preparation method thereof
The invention discloses a traditional Chinese medicine anti-inflammatory preparation for a nasal cavity and a preparation method of the traditional Chinese medicine anti-inflammatory preparation, and belongs to the technical field of pharmacy of traditional Chinese medicines. The traditional Chinese medicine anti-inflammatory preparation disclosed by the invention comprises the following raw materials in percentage by mass: 0.1%-20% of fulvate, 5%-20% of honeysuckle flower, 1%-5% of liquorice, 1%-5% of fructus forsythia, 1%-15% of radix angelicae, 1%-15% of asarum, 1%-10% of common alstonia leaf, 1%-5% of borneol, 1%-5% of a mint extract and the balance of conventional auxiliary materials. The traditional Chinese medicine anti-inflammatory preparation disclosed by the invention can be prepared into a nasal spray, a gel and a nasal drop; and nasal and nasal sinus inflammatory diseases are locally treated. The traditional Chinese medicine anti-inflammatory preparation disclosed by the invention has the characteristics of fast acting, accurate curative effect, good compliance, mild medicine property, and is safe and reliable; and clinical application and patient application are facilitated.
Owner:KUNMING UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com