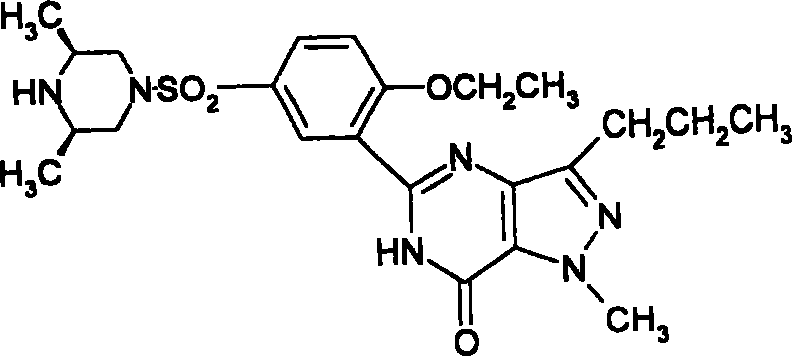
Medicinal composition for treating impotence
A composition and drug technology, applied in the directions of drug combination, drug delivery, drug formulation, etc., can solve the problem of no corresponding prompts, no indication of the therapeutic effect of idenafil dose, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1. Determination of Edina nonspecificity
[0037] cGMP Enzymeimmunoassay Biotrak TM (EIA) System (Amersham company RPN226, 43A, W306740) detects different concentrations of edenafil citrate in vitro (10 -4 mol / L, 10 -5 mol / L, 10 -6 mol / L, 10 -7 mol / L, 10 -8 mol / L, 10 -9 mol / L, 10 -10 mol / L, 10 -11 mol / L, 10 -12 mol / L, prepared from white crystalline powder of ardenafil citrate with a content of 99.99%) to inhibit the degradation of cGMP by PDE3A, PDE3B, PDE5 and PDE6.
[0038] Determination of the Inhibitory Effect of Aldenafil Citrate on PDE3A and PDE3B
[0039] Draw a certain amount of human PDE3A (Orbigen, Cat No: bvc-10185, Lot No.10689) and human PDE3B (Orbigen, Cat No: bvc-10186, Lot No.10690), respectively, and dilute the corresponding volume of PBS to make the final concentration 1u / μl. 3u of PDE3A and PDE3B were co-acted with 3200 fmol (50 μl) of cGMP. After acting at 30°C for 20 minutes, 100 μl was added to the assay well, and 100 μl of an...
Embodiment 2
[0050] Example 2: Effects of Aldenafil on Penile Erectile Function Caused by Electrical Stimulation of Peripheral Pelvic Nerves in Anesthetized Dogs
[0051] Standard methods known to those skilled in the art were used to study the effect of edenafil on penile erectile function induced by electrical stimulation of peripheral pelvic nerves in anesthetized dogs. The results showed that, under the condition of no stimulation, edenafil had no obvious effect on intracavernous pressure. Pelvic nerve stimulation (PNS) in the range of 2.5-20Hz, aldenafil 0.3, 1, 3mg / kg can significantly increase penile erectile function, increase penile intracavernosal pressure (ICP), about 30-90 minutes after administration The effect reached its peak. And there is an obvious dose-effect relationship on the effect of electrical stimulation on the peripheral pelvic nerve of anesthetized dogs to cause penile erection. At 2.5Hz, the maximum increase percentages of erectile function index of the three ...
Embodiment 3
[0054] Embodiment 3: clinical efficacy test
[0055] A randomized, double-blind, multi-center, parallel controlled trial method was used to study the clinically effective dosage of different doses of Aldenafil citrate tablets (30mg, 60mg) and placebo in the treatment of male erectile dysfunction, and to further evaluate its safety and efficacy.
[0056]Methods: This clinical trial was carried out in 5 national clinical base test centers, and a total of 250 patients were randomly selected, including 84 cases in the 30mg group and 83 cases in the 60mg group. All 250 subjects, after a 4-week screening preparation period, were randomly and double-blindly entered into 3 parallel dose groups (30mg, 60mg and placebo groups), and received 4 weeks of treatment. The main evaluation indicators of effectiveness were the erectile function score in IIEF at the end of treatment, the style of the patient's sexual life diary (SEP) and the percentage of 3 answers "yes". The safety evaluation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com