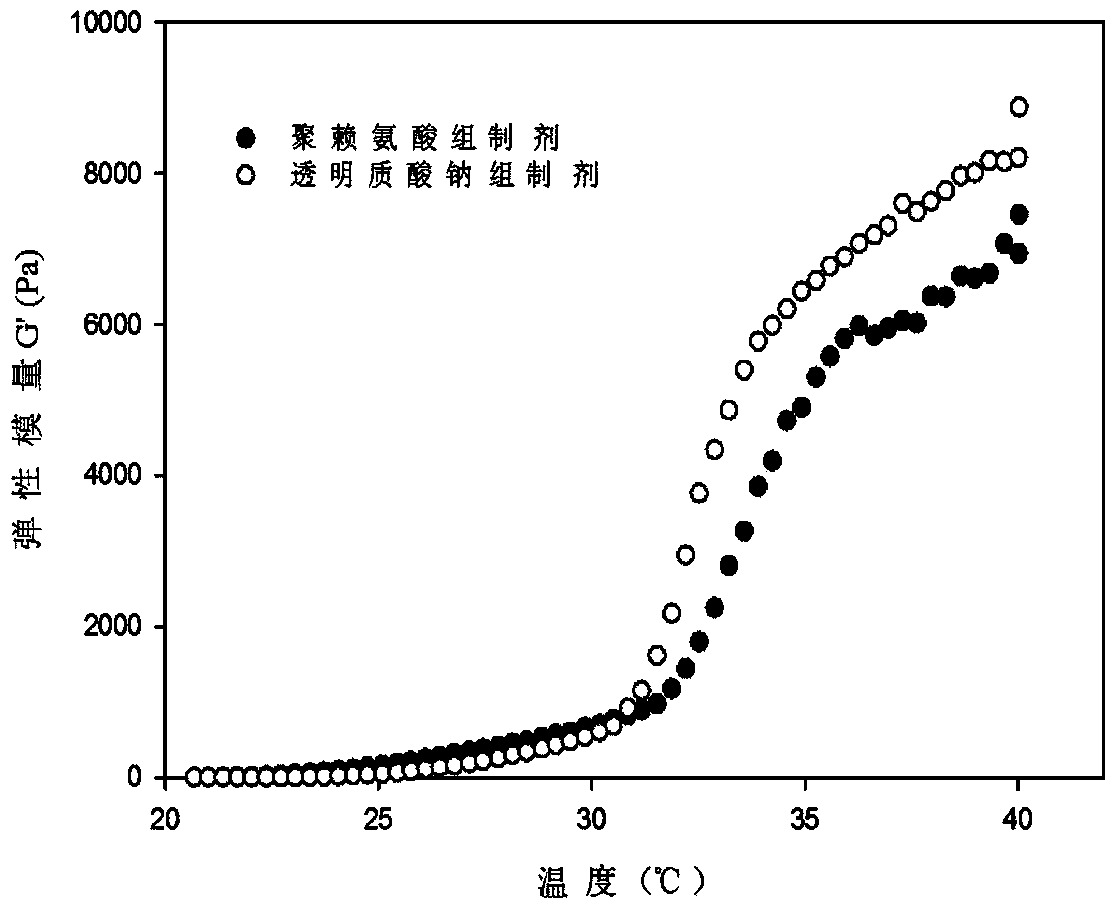
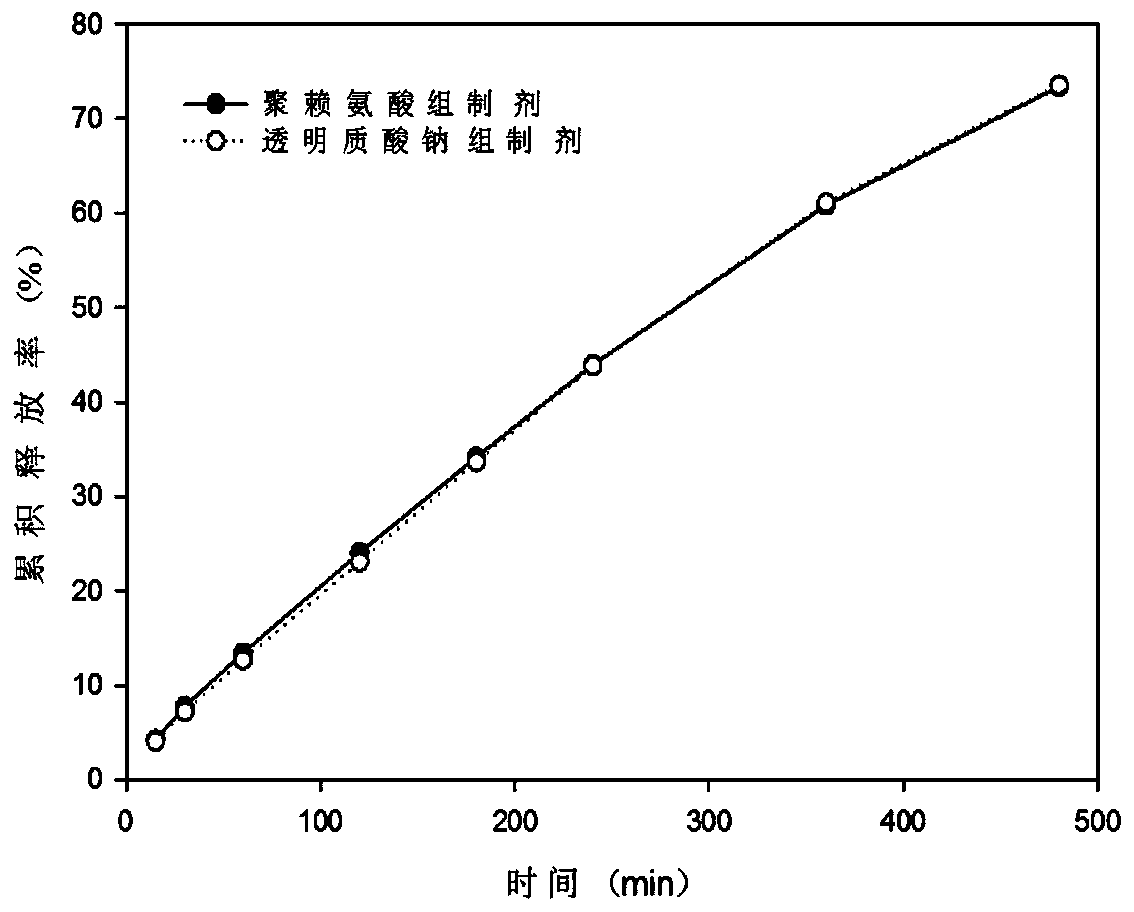
Nose temperature-sensitive type hydrogel spray
A temperature-sensitive, hydrogel technology, used in allergic diseases, aerosol delivery, medical preparations with inactive ingredients, etc. Extended residence time, less irritating, easy-to-obtain results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Phenylephrine Hydrochloride Nasal Thermosensitive Gel Spray I
[0030] Weigh 1.0% phenylephrine hydrochloride and 0.5% polylysine, add to the prescribed amount of water while stirring, dissolve and cool at 4°C. Weigh 0.5% poloxamer 188 and 17% poloxamer 407, and add them into the above cooled solution while stirring. After stirring well, keep it at 4°C until a clear solution without bubbles is formed. Wherein each component weight percent is as follows:
[0031]
Embodiment 2
[0032] Example 2 Phenylephrine Hydrochloride Nasal Thermosensitive Gel Spray II
[0033] Weigh 1.0% phenylephrine hydrochloride, 0.5% sodium hyaluronate (MW: 11000Da), 0.5% methylparaben, add to the prescribed amount of water while stirring, dissolve and cool at 4°C. Weigh 0.8% poloxamer 188 and 17% poloxamer 407, add to the above cooled solution while stirring, after stirring thoroughly, keep it at 4°C until a clear solution without bubbles is formed. Wherein each component weight percent is as follows:
[0034]
Embodiment 3
[0035] Embodiment 3 xylometazoline hydrochloride nasal temperature-sensitive gel spray
[0036] Weigh 0.1% xylometazoline hydrochloride and 1.5% sodium hyaluronate (MW: 11000Da), add to the prescribed amount of water while stirring, dissolve and cool at 4°C. Weigh 1.5% poloxamer 188 and 18% poloxamer 407, add to the above cooled solution while stirring, after stirring thoroughly, keep it at 4°C until a clear solution without bubbles is formed. Wherein each component weight percent is as follows:
[0037]
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com