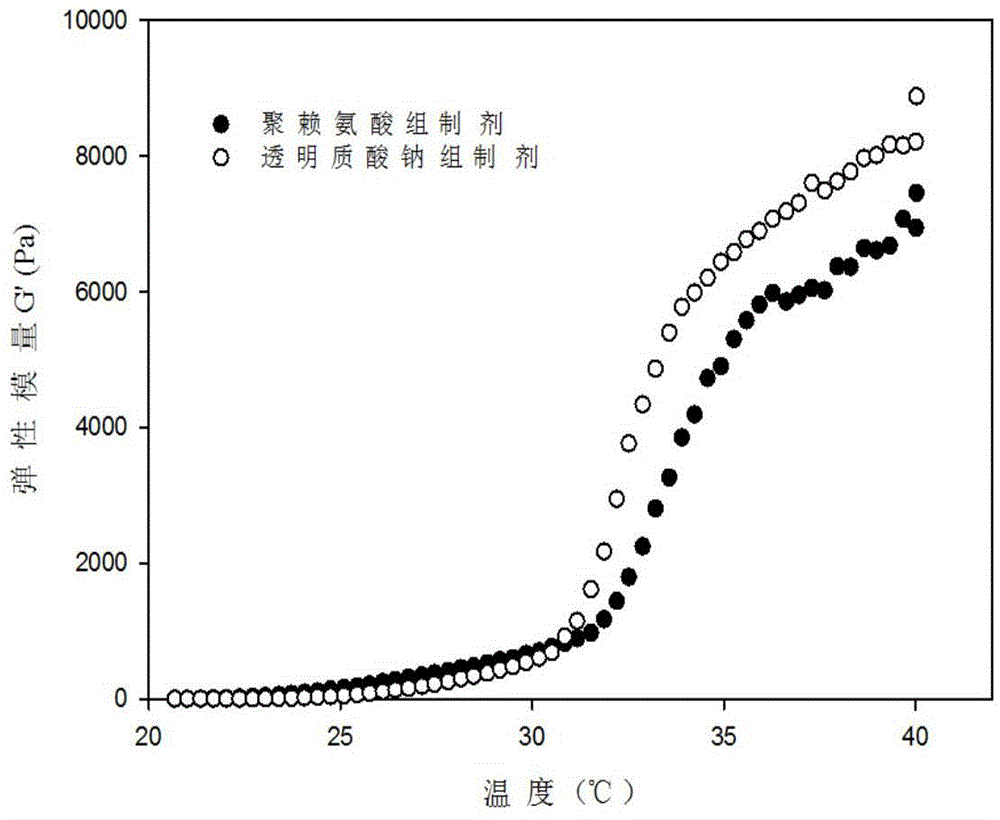
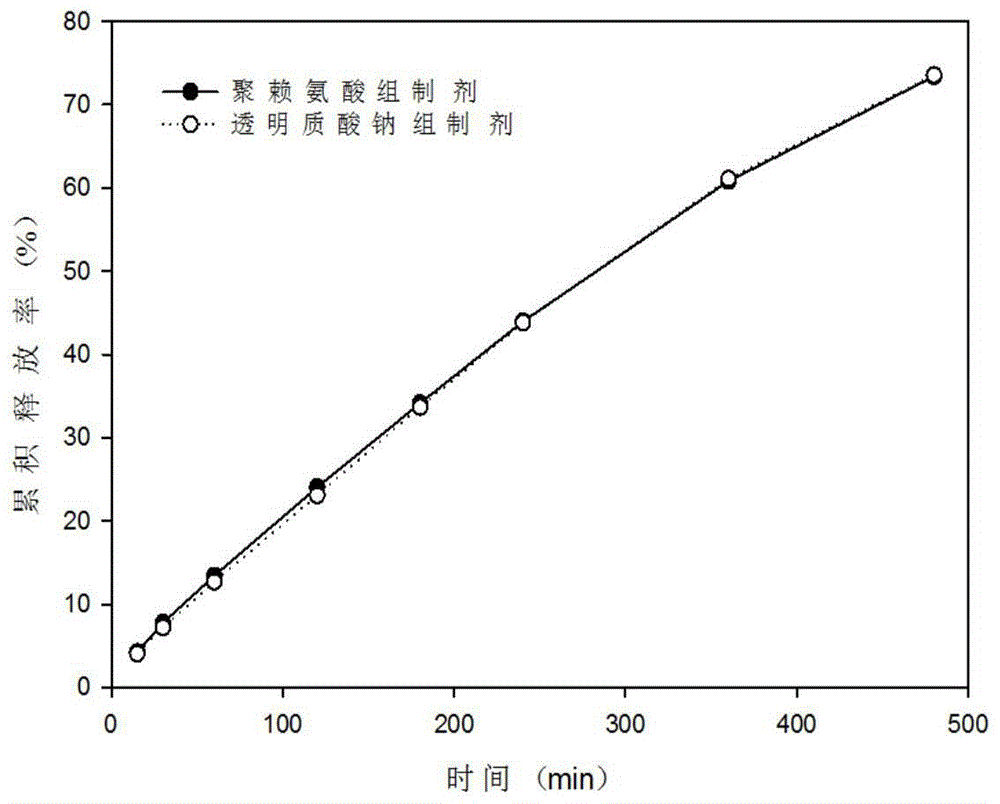
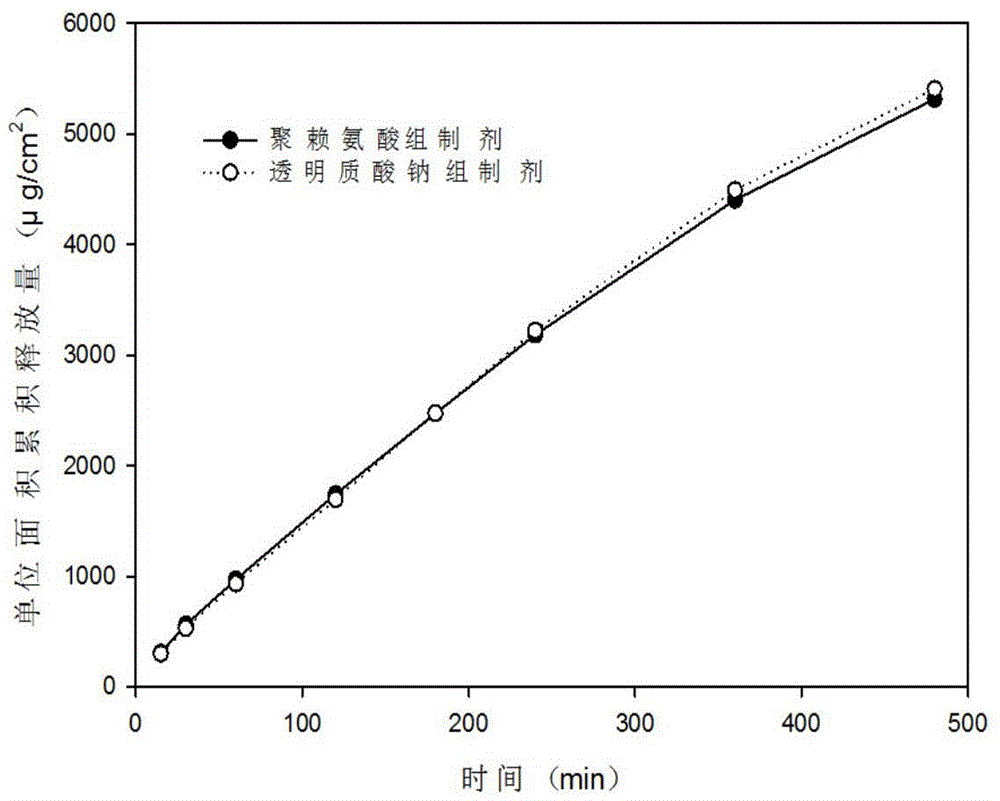
Nasal temperature-sensitive type hydrogel spray
A temperature-sensitive, hydrogel technology, applied in the direction of aerosol delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve nasal cilia recovery obstacles, high strength, and foreign body sensation in the nasal cavity and other problems, to achieve the effect of prolonging the residence time, less irritation, and easy acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Phenylephrine Hydrochloride Nasal Thermosensitive Gel Spray I
[0030] Weigh 1.0% phenylephrine hydrochloride and 0.5% polylysine, add to the prescribed amount of water while stirring, dissolve and cool at 4°C. Weigh 0.5% Poloxamer 188 and 17% Poloxamer 407, add them into the above-mentioned cooled solution while stirring, and after fully stirring, keep it at 4°C until a clear solution without bubbles is formed. Wherein each component weight percent is as follows:
[0031]
Embodiment 2
[0032] Example 2 Phenylephrine Hydrochloride Nasal Thermosensitive Gel Spray II
[0033] Weigh 1.0% phenylephrine hydrochloride, 0.5% sodium hyaluronate (MW: 11000Da), 0.5% methylparaben, add to the prescribed amount of water while stirring, dissolve and cool at 4°C. Weigh 0.8% poloxamer 188 and 17% poloxamer 407, add them into the above-mentioned cooled solution while stirring, after fully stirring, keep at 4°C until a clear solution without bubbles is formed. Wherein each component weight percent is as follows:
[0034]
Embodiment 3
[0035] Example 3 Xylometazoline Hydrochloride Nasal Thermosensitive Gel Spray
[0036] Weigh 0.1% xylometazoline hydrochloride and 1.5% sodium hyaluronate (MW: 11000Da), add to the prescribed amount of water while stirring, dissolve and cool at 4°C. Weigh 1.5% poloxamer 188 and 18% poloxamer 407, and add them into the above cooled solution while stirring. After fully stirring, keep it at 4°C until a clear solution without bubbles is formed. Wherein each component weight percent is as follows:
[0037]
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com