Basic fibroblast growth factor nasal spray for treating Alzheimer's disease
A nasal spray, growth factor technology, applied in basic fibroblast growth factor nasal spray, basic fibroblast growth factor nasal spray for Alzheimer's disease and its preparation field, can solve the problem of bFGF nasal cavity which has not yet been seen Problems such as sprays, to achieve good application prospects, convenient administration, and reduce side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Prepare bFGF nasal spray with chitosan as absorption enhancer according to Table 1.
[0052] Table 1b FGF nasal spray prescription (w / v%)
[0053]
[0054]
[0055] (1) Preparation method: take a buffer solution of about 80% of the prescription amount, add chitosan, make it fully swell until it is completely dissolved; add preservatives, isotonic regulators, and anti-adsorbents, stir and dissolve to obtain liquid a; Dissolve the prescribed amount of bFGF in a small amount of buffer solution, add a stabilizer and mix to obtain liquid b; mix liquid a and liquid b, add buffer solution to the full amount, filter and sterilize with a 0.22 μm microporous membrane, and pack it into a quantitative nasal spray In the device, the specification of each bottle is 5ml;
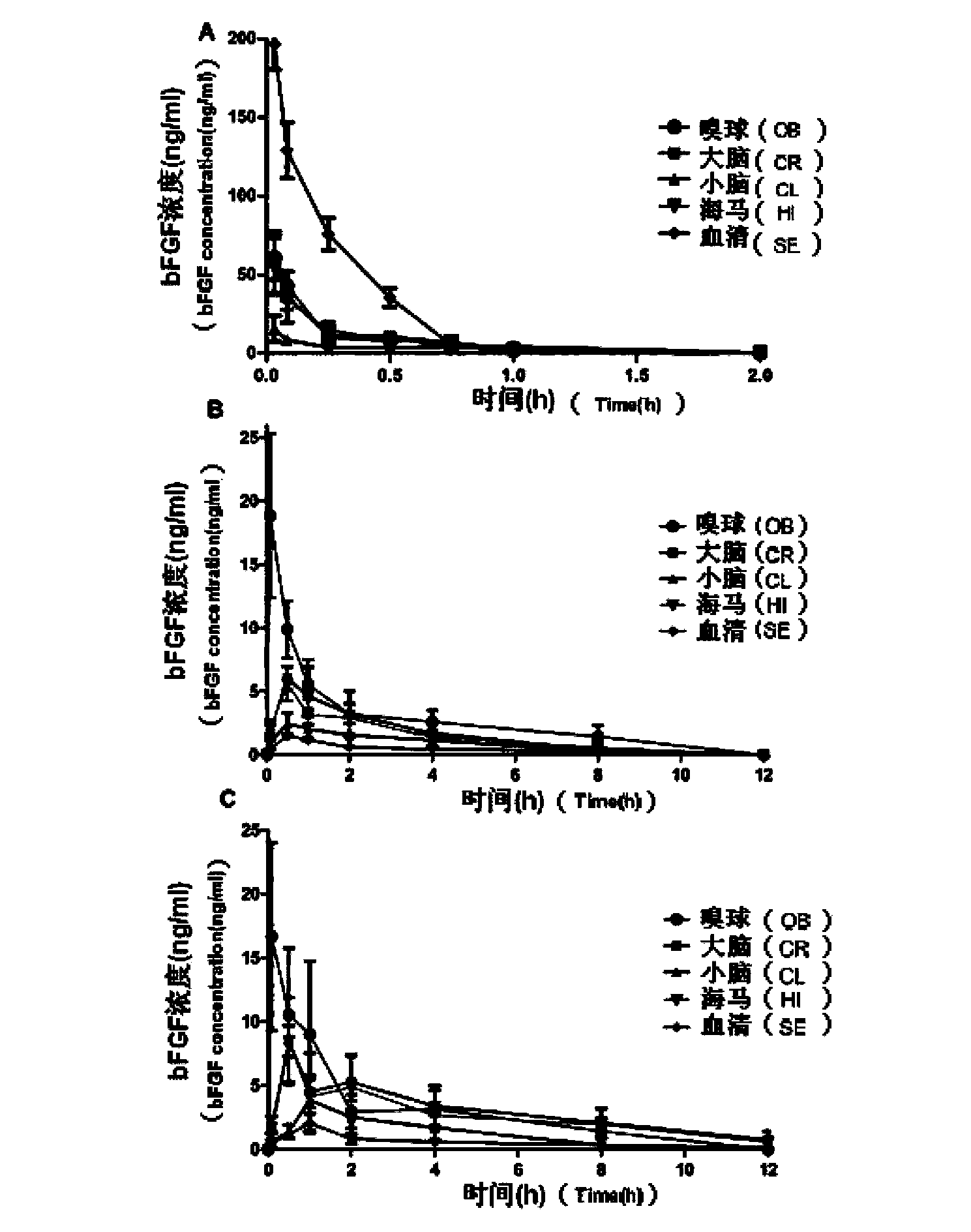
[0056] (2) Stability test: Take the rabbit nasal mucosa homogenate, add the preparation obtained in Example 1 and bFGF solution (dissolve the drug directly in the buffer), incubate at 37°C for 4 hours...
Embodiment 2
[0063] Prepare bFGF nasal spray with cyclodextrin as absorption enhancer according to Table 3.
[0064] Table 3bFGF nasal spray prescription (w / v%)
[0065]
[0066] (1) Preparation method: Take a buffer solution with about 80% of the prescription amount, add a bioadhesive agent to make it fully swell until it is completely dissolved; add cyclodextrin in stages, stir to dissolve, and then add preservatives, isotonic regulators, Anti-adsorbent, stir and dissolve to obtain liquid a; dissolve the prescribed amount of bFGF in a small amount of buffer solution, add stabilizer and mix to obtain liquid b; mix liquid a and liquid b, add buffer solution to the full amount, and filter through 0.22μm microporous Sterilized by membrane filtration, packed in a quantitative nasal spray device, the size of each bottle is 5ml;
[0067] (2) The stability investigation method is the same as in Example 1, and the results are shown in Table 4. In addition to the stabilizer, cyclodextrin can a...
Embodiment 3
[0077] Prepare bFGF nasal spray with Tween, poly-L-Arg and sodium caprate as absorption enhancer according to Table 6.
[0078] Table 6bFGF nasal spray prescription (w / v%)
[0079]
[0080] (1) preparation method is with above-mentioned embodiment 2;
[0081] (2) The investigation method of ciliary toxicity is the same as that of the above-mentioned Example 1, and the percentages of ciliary continuous movement of each preparation in this embodiment are calculated. The results are shown in Table 7. Prescriptions 12 to 17 have only weak ciliary toxicity;
[0082] Nasal cilia toxicity of table 7bFGF nasal spray (mean±SD, n=4)
[0083]
[0084] * p<0.05, there was a significant difference in the normal saline group;
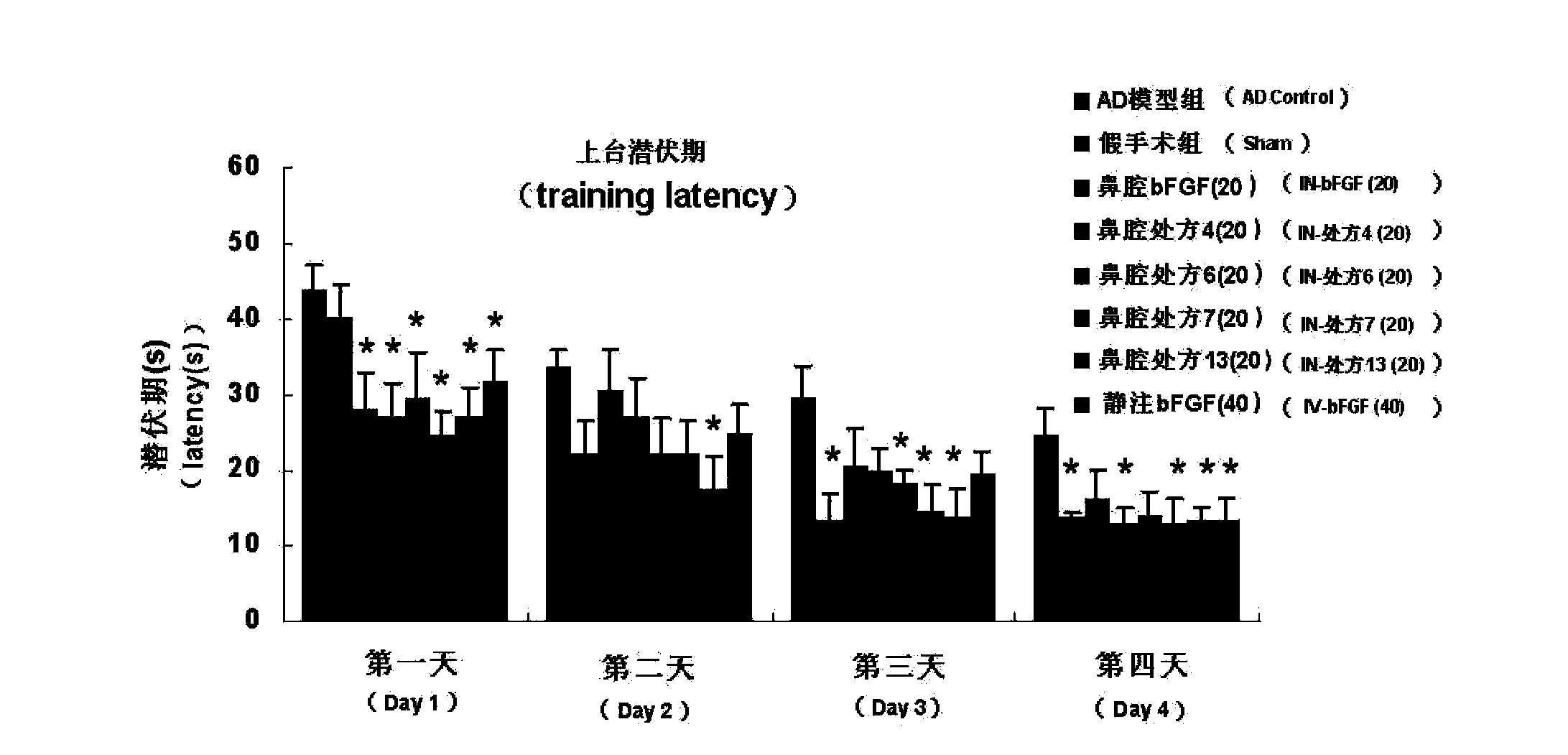
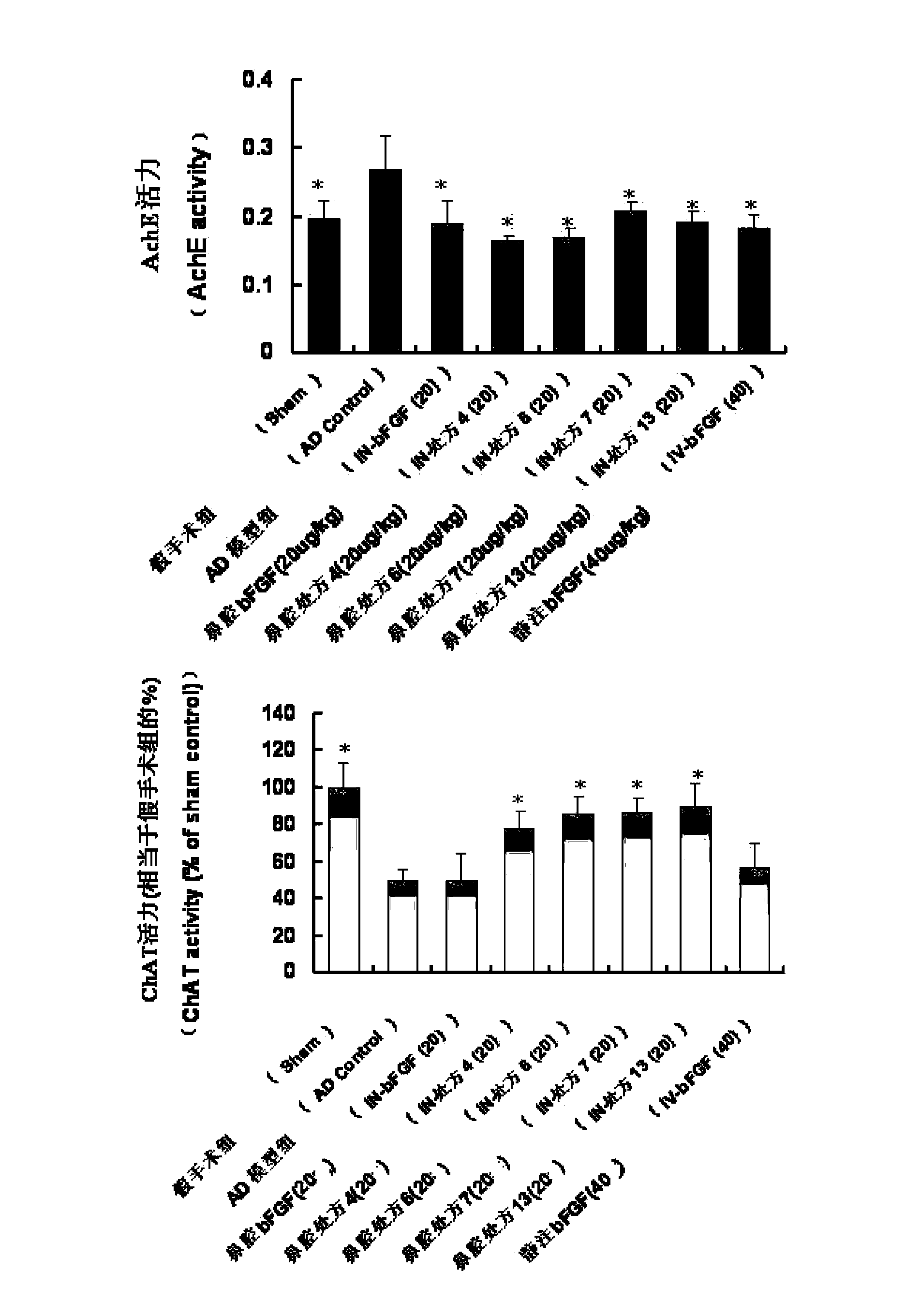
[0085] (3) The research method of nasal absorption was the same as that of the above-mentioned Example 1; the results showed that after adding various absorption enhancers, the nasal absorption of bFGF was significantly increased, and its AUC was 1.27-2.65 t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com