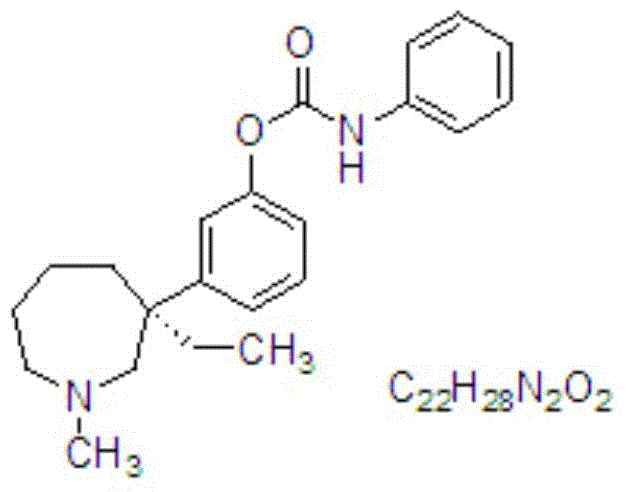
Nasal spray with cholinesterase inhibitors XQ528
A nasal spray, cholinesterase technology, applied in aerosol delivery, non-active components of polymer compounds, nervous system diseases, etc., can solve problems such as instability of XQ528, improve spatial memory impairment, and achieve good nasal absorption. , the effect of raising the level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Prepare the XQ528 nasal cavity solution with cyclodextrin as a stabilizer according to Table 1, and fill it in a nasal spray device, with a volume of 3-5 ml per bottle.
[0040] Table 1XQ528 Nasal Solution Spray Prescription (w / v%)
[0041]
[0042]
[0043] Table 1 (continued table) XQ528 nasal cavity solution type spray prescription (w / v%)
[0044]
[0045] (1) The preparation process is as follows: take about 60% of the buffer solution of the prescription amount in the preparation container, add bioadhesive agent to make it fully swell until completely dissolved; add bacteriostatic agent, isotonic regulator, metal ion chelating agent and stir Dissolve; add cyclodextrin in stages, stir to dissolve, then add XQ528, stir for 1-2 hours, add buffer to the full amount after complete dissolution, and mix well. After the pH and content are qualified, the liquid medicine is filtered through a 0.45 μm microporous membrane until it is clear, and then sterilized by fil...
Embodiment 2
[0049] Prepare XQ528 nasal cavity solution with polyethylene glycol (PEG) as a stabilizer according to Table 2, and fill it in a nasal spray device, and the amount of each bottle is 3-5 ml.
[0050] Table 2XQ528 nasal cavity solution type spray prescription (w / v%)
[0051]
[0052] (1) The preparation process is as follows: same as Example 1, except that cyclodextrin is replaced with PEG.
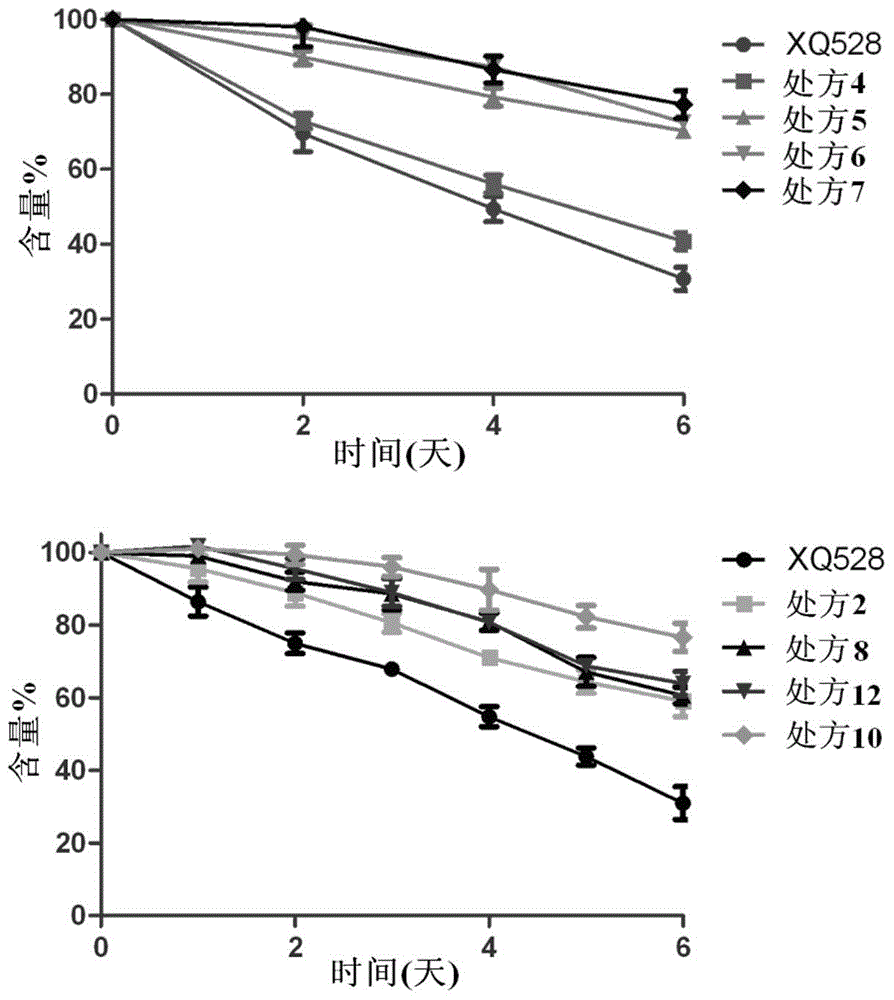
[0053] (2) Thermal stability investigation: The thermal stability of the formulation obtained in Example 2 was investigated by the same method as in Example 1. As a result, after the pure XQ528 solution was placed at 60°C for 6 days, the remaining dose was only 30.79±3.05% of the initial dose. After adding PEG as a stabilizer, the stability of the solution was significantly improved, and the remaining dose of XQ528 after 6 days was the initial dose. 40% to 64% of that. Figure 4 is the percentage-time curve of the remaining drug dose of prescriptions 14, 16 and 18.
Embodiment 3
[0055] Prepare the ion-sensitive nasal cavity instant gel according to Table 3, and fill it in a nasal spray device, and the volume of each bottle is 3-5ml.
[0056] Table 3 Prescription (w / v%) of ion-sensitive nasal cavity instant gel spray
[0057]
[0058] Table 3 (continued table) prescription (w / v%) of ion-sensitive nasal cavity instant gel spray
[0059]
[0060]
[0061] (1) The preparation process is as follows: take triple-distilled water with about 60% of the prescription amount, adjust the pH to about 5.5 with 0.1N HCl, add a hydrophilic gel material, and leave it at room temperature or heat (depending on the nature of the material) to make it Fully swell until completely dissolved; add bacteriostatic agent and isotonic regulator and stir to dissolve; add cyclodextrin and XQ528, stir until a clear solution is obtained, adjust the pH to 3.8-5.5 with 0.1N HCl, and finally add triple distilled water to the full amount, Mix well. After the pH and content are ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com