H102 peptide nasal solution type spray for treatment of Alzheimer's disease
A nasal spray and Alzheimer's disease technology, applied in the field of H102 peptide nasal solution spray and its preparation, can solve the problems of poor mucous membrane penetration and instability of polypeptide drugs, so as to increase the dosage and benefit the prevention and treatment , the effect of convenient administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] According to Table 1, the H102 peptide nasal solution spray with chitosan as an absorption enhancer was prepared.
[0062] Table 1 Prescription of H102 peptide nasal solution spray (w / v %)
[0063]
[0064] ;
[0065] (1) The preparation process is as follows: take a buffer solution with about 70% of the prescription amount, add chitosan to make it fully swell until it is completely dissolved; then add antibacterial agent, stabilizer, osmotic pressure regulator, freeze-dried scaffold agent, Stir the anti-adsorbent and H102 peptide to fully dissolve, add buffer to the full amount; the liquid is sterilized by filtration through a 0.22 μm microporous membrane, and then packed into sterilized nasal spray bottles for freeze-drying; after freeze-drying, take out , Add a bottle cap with a quantitative spray pump, and store in an airtight low temperature. Before use, add sterilized three-distilled water to dissolve and then spray it for administration;
[0066] (2) Stab...
Embodiment 2
[0073] According to Table 3, the H102 peptide nasal cavity solution spray with cyclodextrin and Tween as absorption accelerators was prepared.
[0074] Table 3 bFGF nasal spray prescription (w / v %)
[0075]
[0076] ;
[0077] (1) The preparation process is as follows: take a buffer solution of about 70% of the prescription amount, add a bioadhesive agent to make it fully swell until it is completely dissolved; then add an absorption enhancer, a bacteriostat, a stabilizer, an osmotic pressure regulator, Dry the scaffolding agent, anti-adsorbent and H102 peptide, stir to fully dissolve, add buffer to the full amount; the drug solution is sterilized by filtering through a 0.22 μm microporous membrane, and then packed into sterilized nasal spray bottles to freeze-dry; freeze-dry After completion, take out and add the bottle cap with a quantitative spray pump, and store in airtight low temperature; before use, add sterilized three-distilled water to dissolve and then spray i...
Embodiment 3
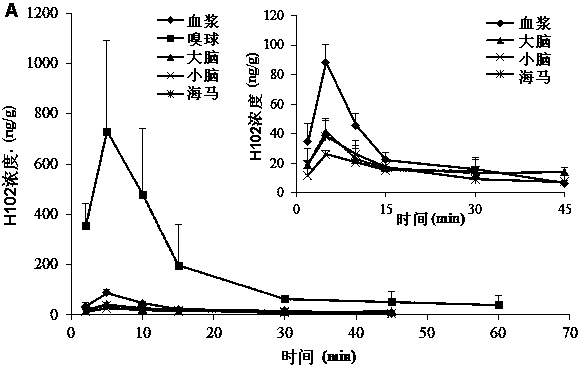
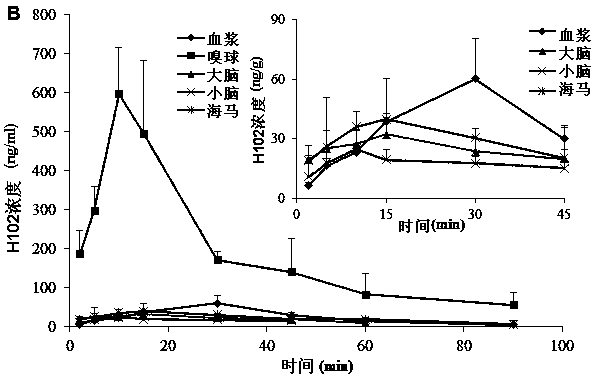
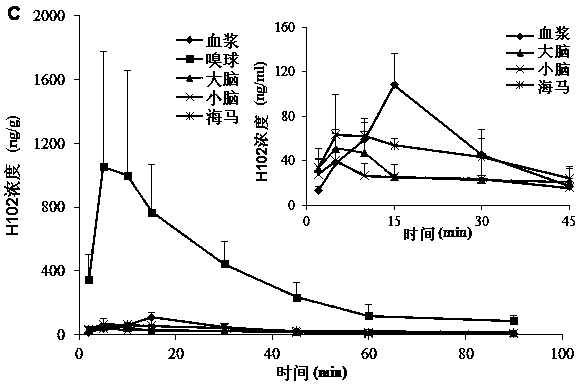
[0080] SD rats were anesthetized, respectively nasal administration of H102 peptide solution, prescriptions 2, 3, 5 in the above-mentioned embodiment 1 and 7, 9, 10, 13 in the above-mentioned embodiment 2, and tail vein injection of the H102 peptide solution (the medicine was directly dissolved in buffer), wherein the nasal administration dose is 10 times that of intravenous injection; blood is taken from the tail vein at a predetermined time point, and plasma is obtained by centrifugation; after treatment, the concentration of H102 peptide is determined by LC-MS method, and the drug-time curve is calculated by trapezoidal method The area AUC 0-t , the results are shown in Table 4 (AUC values in blood after nasal cavity and intravenous injection), the H102 peptide itself has poor ability to penetrate the mucosa, and the drug concentration in the blood was not measured after nasal cavity administration; however, after adding an absorption enhancer, it increased significantly ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com