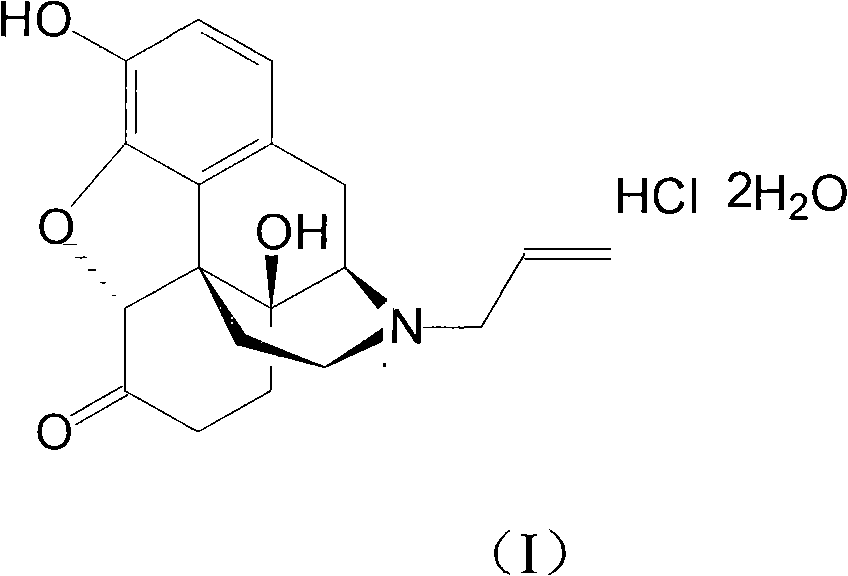
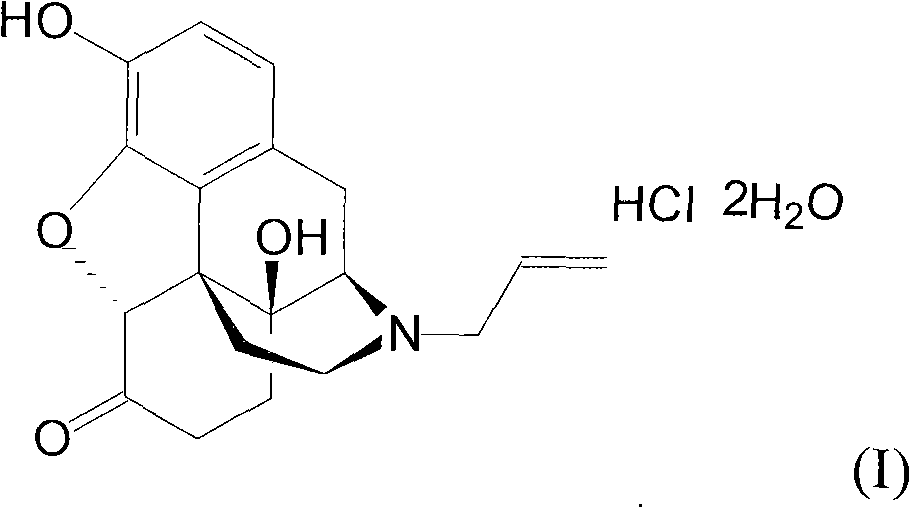
Naloxone hydrochloride compound with high purity
A technology for naloxone hydrochloride and crude naloxone hydrochloride, applied in the field of medicine, can solve the problems of affecting clinical application, poor purity, unqualified preparation quality, etc., and achieve the effects of low cost, improved purity, and optimized product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The refining of embodiment 1 naloxone hydrochloride
[0029] (1) Dissolve 100g of crude naloxone hydrochloride in 1000ml of water, stir for 10 minutes, slowly add 5% sodium hydroxide solution until the pH value is 9.5, precipitate insoluble matter, stir for 20 minutes, filter, and dry under reduced pressure at 50°C , Denaloxone 75.3g;
[0030] (2) Naloxone 10g is packed into 200ml polyamide resin, at first carry out eluting with 10% aqueous ethanol (V / V), remove the first component that column elution separates, then use 80% aqueous ethanol ( V / V) further elutes again, obtains the second component, collects the second eluting component that separates most on the post;
[0031] (3) Add the collected components into 200ml of dichloromethane, add 0.3g of activated carbon, heat to 50°C, stir and adsorb for 15 minutes, filter for decarburization, and collect the filtrate;
[0032] (4) In the filtrate, the hydrochloric acid solution of 0.5mol / L is added dropwise to a pH val...
Embodiment 2
[0033] The refining of embodiment 2 naloxone hydrochloride
[0034] (1) Dissolve 100g of crude naloxone hydrochloride in 1000ml of water, stir for 10 minutes, slowly add 5% sodium hydroxide solution until the pH value is 9.5, precipitate insoluble matter, stir for 20 minutes, filter, and dry under reduced pressure at 50°C , Denaloxone 74.6g;
[0035] (2) Naloxone 15g is packed into 300ml polyamide resin, at first carry out eluting with 10% aqueous ethanol (V / V), remove the first component that column elution separates, then use 80% aqueous ethanol ( V / V) further elutes again, obtains the second component, collects the second eluting component that separates most on the post;
[0036] (3) Add the collected components into 300ml of dichloromethane, add 0.4g of activated carbon, heat to 40°C, stir and adsorb for 20 minutes, filter for decarburization, and collect the filtrate;
[0037] (4) in the filtrate, dropwise add the hydrochloric acid solution of 1.0mol / L to the pH value ...
Embodiment 3
[0038] The refining of embodiment 3 naloxone hydrochloride
[0039] (1) Dissolve 100g of crude naloxone hydrochloride in 1000ml of water, stir for 10 minutes, slowly add 5% sodium bicarbonate solution until the pH value is 10.0, precipitate insoluble matter, stir for 20 minutes, filter, and dry under reduced pressure at 50°C , Denaloxone 75.2g;
[0040] (2) Naloxone 15g is packed into 300ml polyamide resin, at first carry out eluting with 15% aqueous ethanol (V / V), remove the first component that column elution separates, then use 85% aqueous ethanol ( V / V) further elutes again, obtains the second component, collects the second eluting component that separates most on the post;
[0041] (3) Add the collected components into 300ml of dichloromethane, add 0.4g of activated carbon, heat to 40°C, stir and adsorb for 30 minutes, filter for decarburization, and collect the filtrate;
[0042](4) In the filtrate, add dropwise the hydrochloric acid solution of 2.0mol / L to the pH valu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com