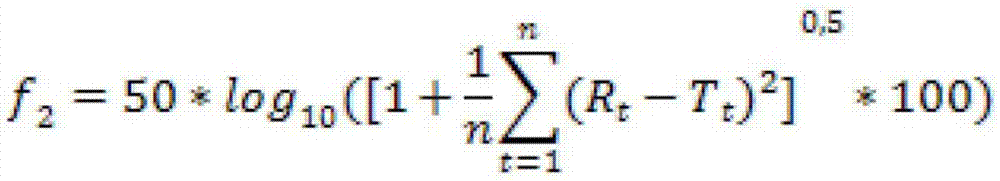Patents
Literature
77 results about "Naloxazone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
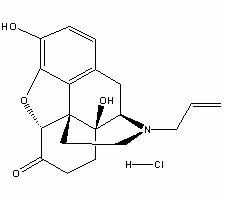
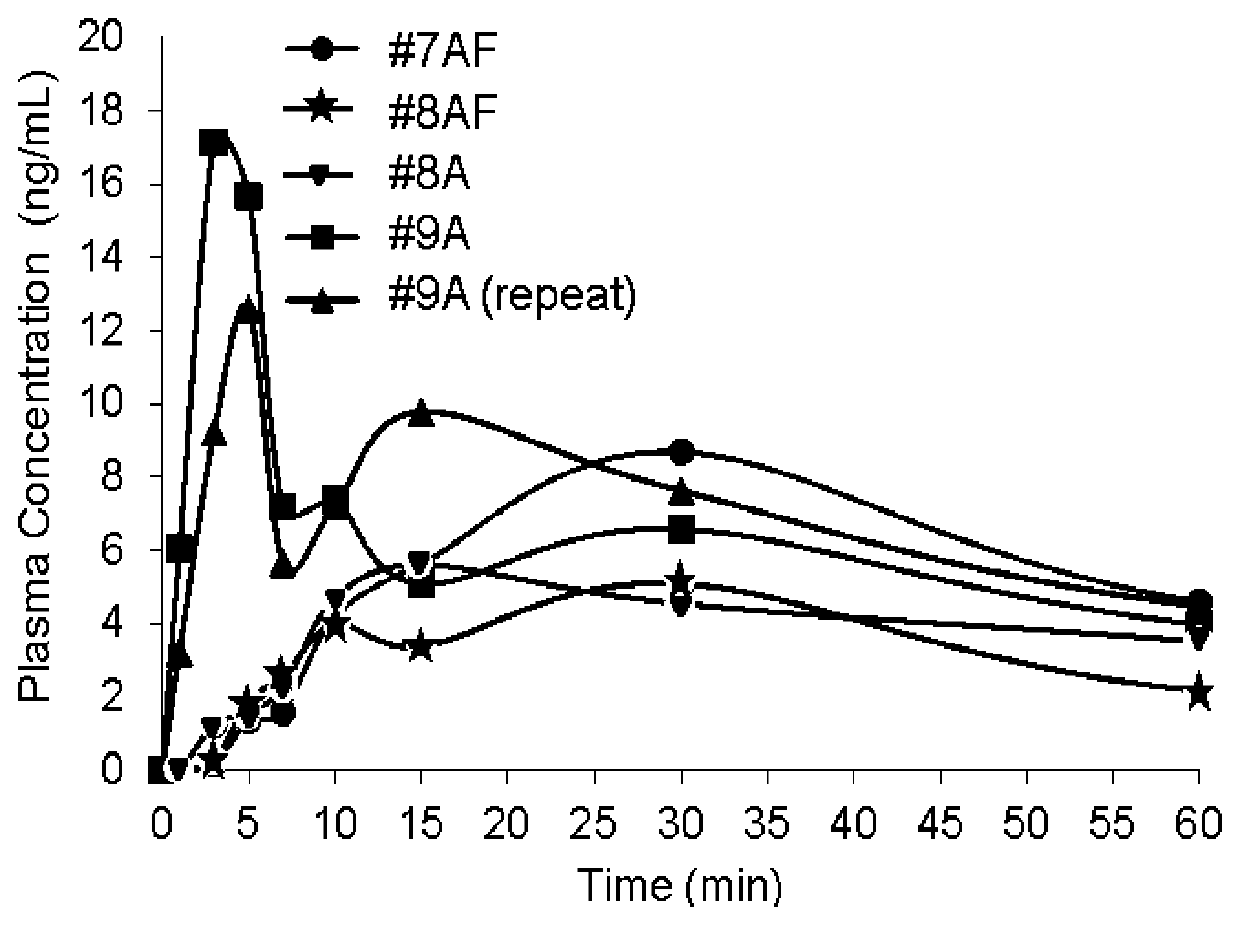
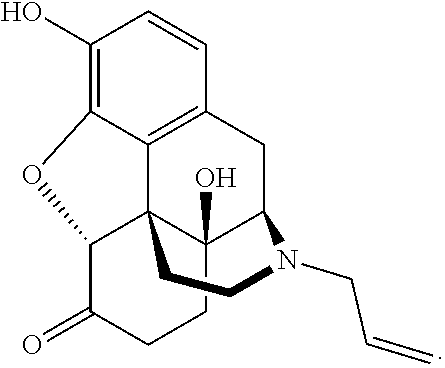
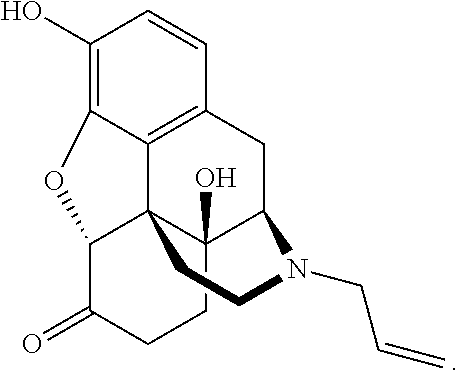
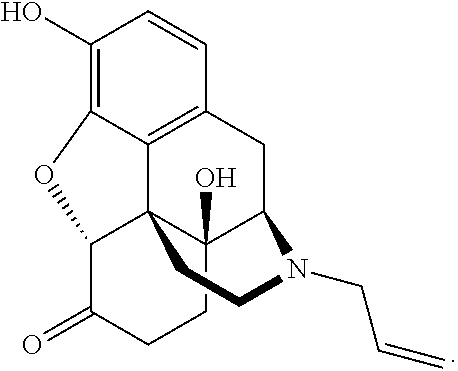
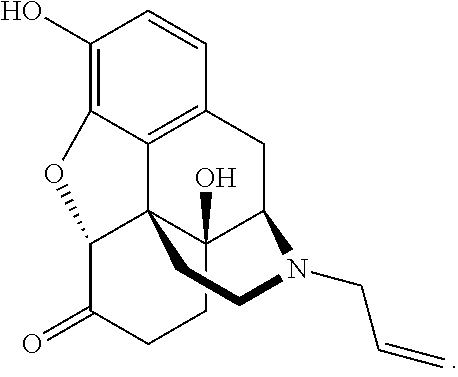
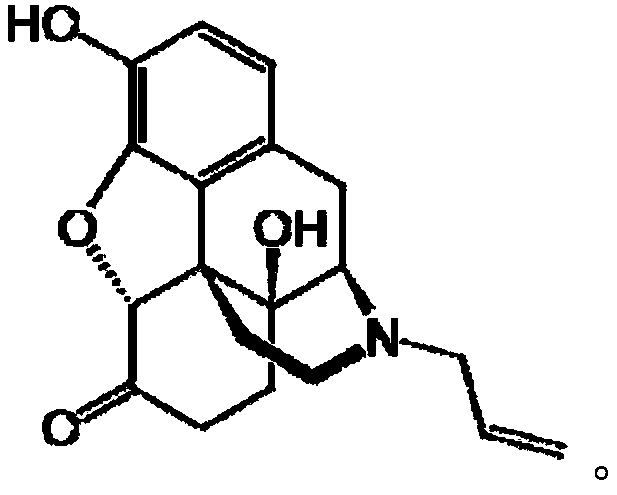
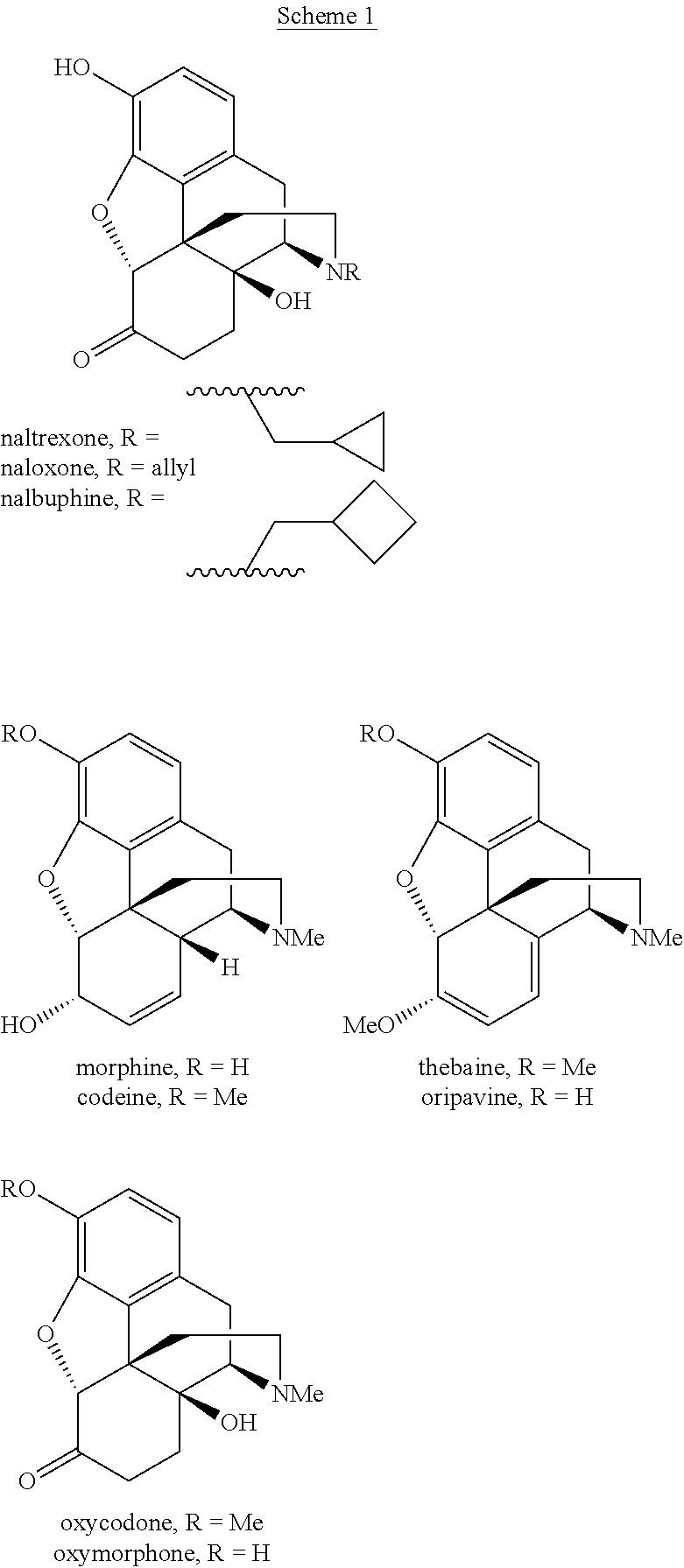
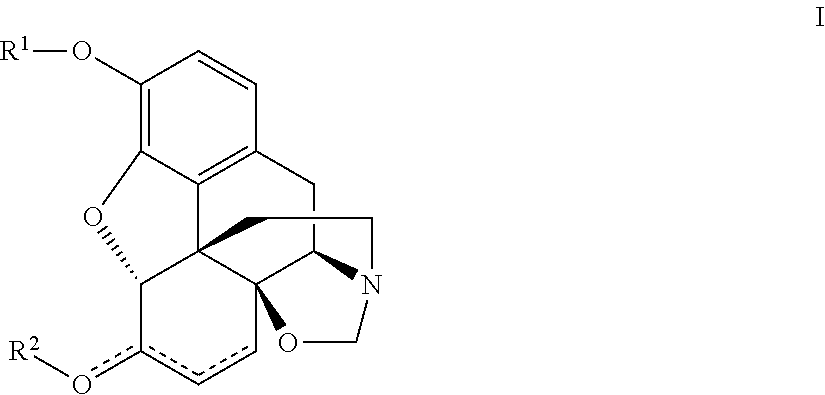
Naloxazone is an irreversible μ-opioid receptor antagonist which is selective for the μ₁ receptor subtype. Naloxazone produces very long lasting antagonist effects as it forms a covalent bond to the active site of the mu-opioid receptor, thus making it impossible for the molecule to unbind and blocking the receptor permanently until the receptor is recycled by endocytosis.
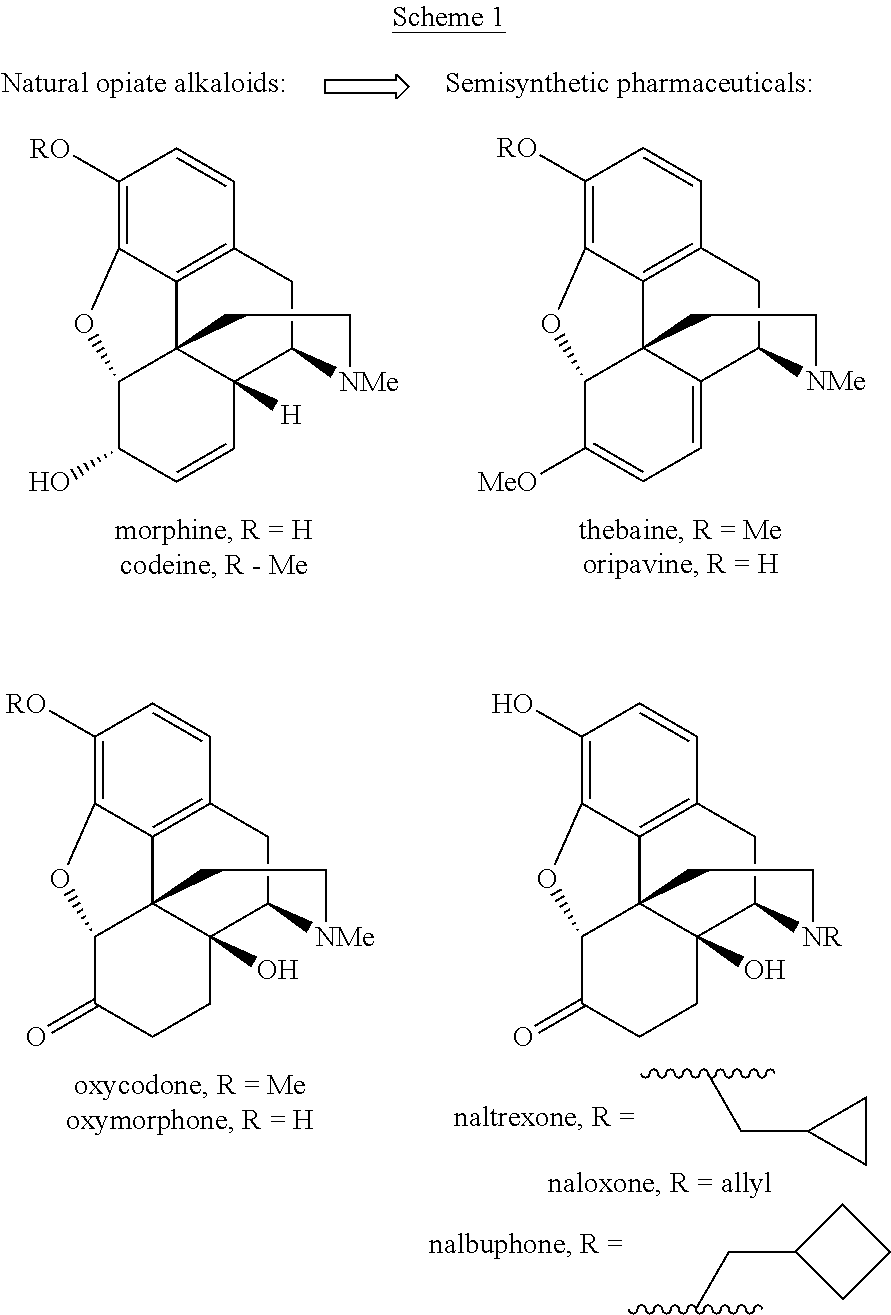
Abuse-resistant controlled-release opioid dosage form
InactiveUS20030065002A1High oral : parenteral potency ratioEliminate the effects ofBiocideNervous disorderControlled releaseOpioid antagonist
Abuse-resistant, controlled release opioid tablets are a combination containing an opioid antagonist such as naloxone at a level that needed to suppress the euphoric effect of the opioid, if the combination were crushed to break the controlled release properties causing the opioid and opioid antagonist to be released as a immediate release product as a single dose. The controlled release nature of the table prevents the accumulation of orally effective amounts of opioid antagonist when taken normally. The opioid antagonist is contained in a controlled-release matrix and released, over time, with the opioid.
Owner:PURDUE PHARMA LP
Analgetic dosage forms that are resistant to parenteral and inhalation dosing and have reduced side effects
InactiveUS20050165038A1Side-effect be reduceEliminate side effectsPowder deliveryBiocideDrugPharmaceutical preservatives
The invention provides a novel solid pharmaceutical dosage form which includes an opiate, an opiate antagonist admixed with the analgetic (opiate agonist) and an amount of a hydrocolloid containing excipient which is effective to form a non-injectable slurry when the dosage form is contacted with water. In addition the dosage form contains pure naloxone in enteric coated form which is designed to release in the colon to prevent or relieve constipation. Thus the formulation, because of the enteric coated naloxone and the hydrocolloid excipient(s), has reduced side effects as compared with formulations which do not contain these features.
Owner:GORDON MAXWELL
Treatment of pain with combinations of nalbuphine and other kappa-opioid receptor agonists and opioid receptor antagonists
InactiveUS20040180916A1Enhances nalbuphine analgesiaMinimize ToxicityBiocideNervous disorderOpioid antagonistPentazocine
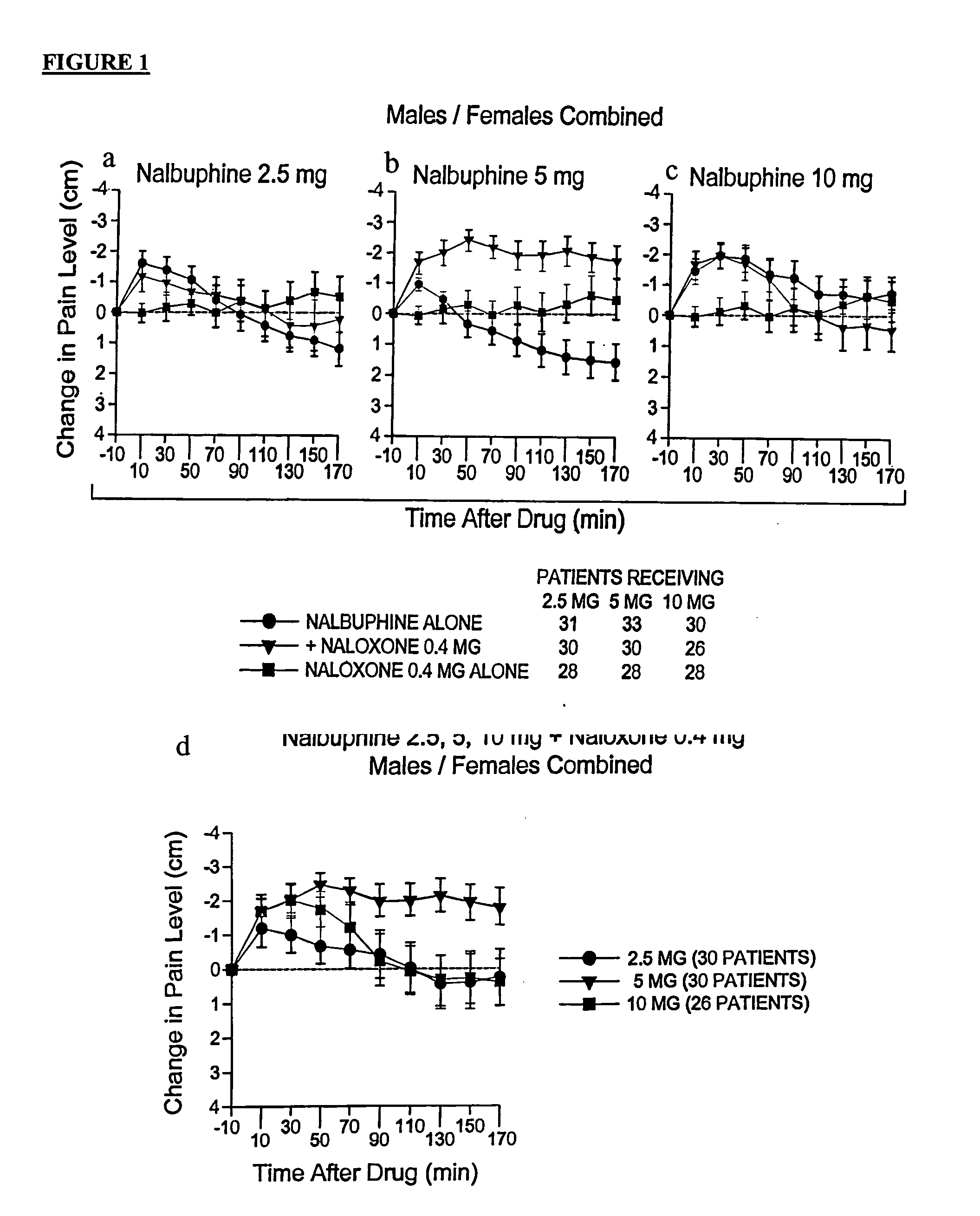
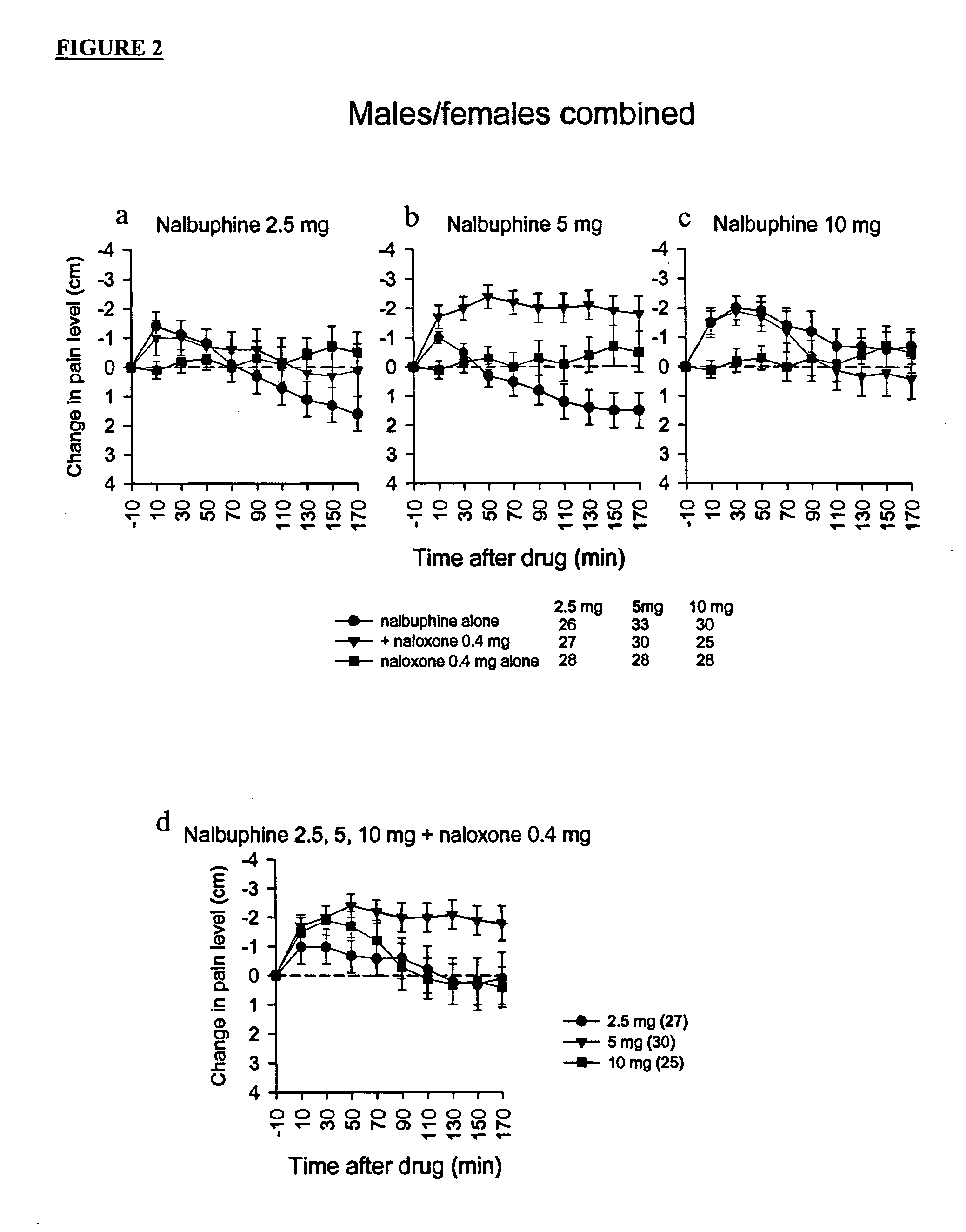
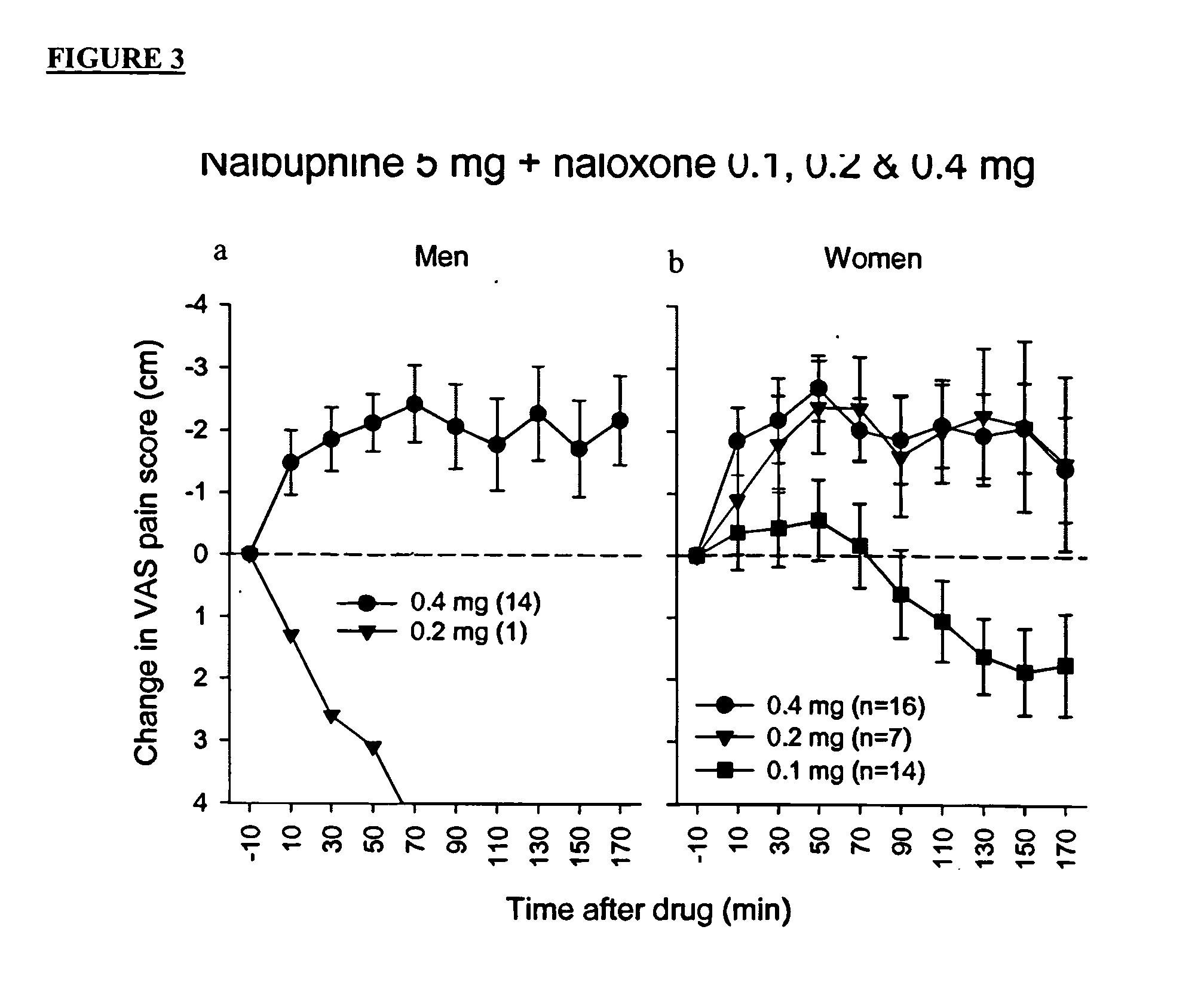
Methods and compositions for treating, managing or ameliorating pain in a subject (preferably, a mammal, and more preferably, a human) comprising administration of a centrally acting (i.e., crosses the blood brain barrier) agonist of a kappa-opioid receptor and a centrally acting opioid antagonist such that the analgesia achieved by this administration is greater than with administration of either the kappa-opioid receptor agonist or the opioid antagonist alone. Preferably the kappa-opioid receptor is nalbuphine or a salt or prodrug of nalbuphine and the opioid antagonist is naloxone or a salt or prodrug of naloxone. Preferred methods of administration include mucosal (e.g. intranasal or pulmonary) and intravenous. Other kappa-opioid receptors include pentazocine and butorphanol.
Owner:RGT UNIV OF CALIFORNIA
Process and compounds for the production of (+)opiates
The invention generally provides processes and intermediate compounds useful for the production of (+)-opiates. Non-limiting examples of (+) opiates that may be derived from one or more compounds of the invention include (+)-noroxymorphone, (+)-naltrexone, (+)-naloxone, (+)-N-cyclopropylmethylnorhydrocodone, (+)-N-cycloproylmethylnorhydromorphone, (+)-N-allylnorhydrocodone, (+)-N-allylnorhydromorphone, (+)-noroxycodone, (+)-naltrexol, (+)-naloxol, and (+)-3-O-methyl-naltrexone.
Owner:SPECGX LLC
Abuse-resistant controlled-release opioid dosage form
InactiveUS20080069881A1High oral : parenteral potency ratioEliminate the effects ofBiocidePowder deliveryOpioid antagonistControl release
Abuse-resistant, controlled release opioid tablets are a combination containing an opioid antagonist such as naloxone at a level above that needed to suppress the euphoric effect of the opioid, if the combination were crushed to break the controlled release properties causing the opioid and opioid antagonist to be released as an immediate release product as a single dose. The controlled release nature of the tablet prevents the accumulation of orally effective amounts of opioid antagonist when taken normally. The opioid antagonist is contained in a controlled-release matrix and released, over time, with the opioid.
Owner:PURDUE PHARMA LP
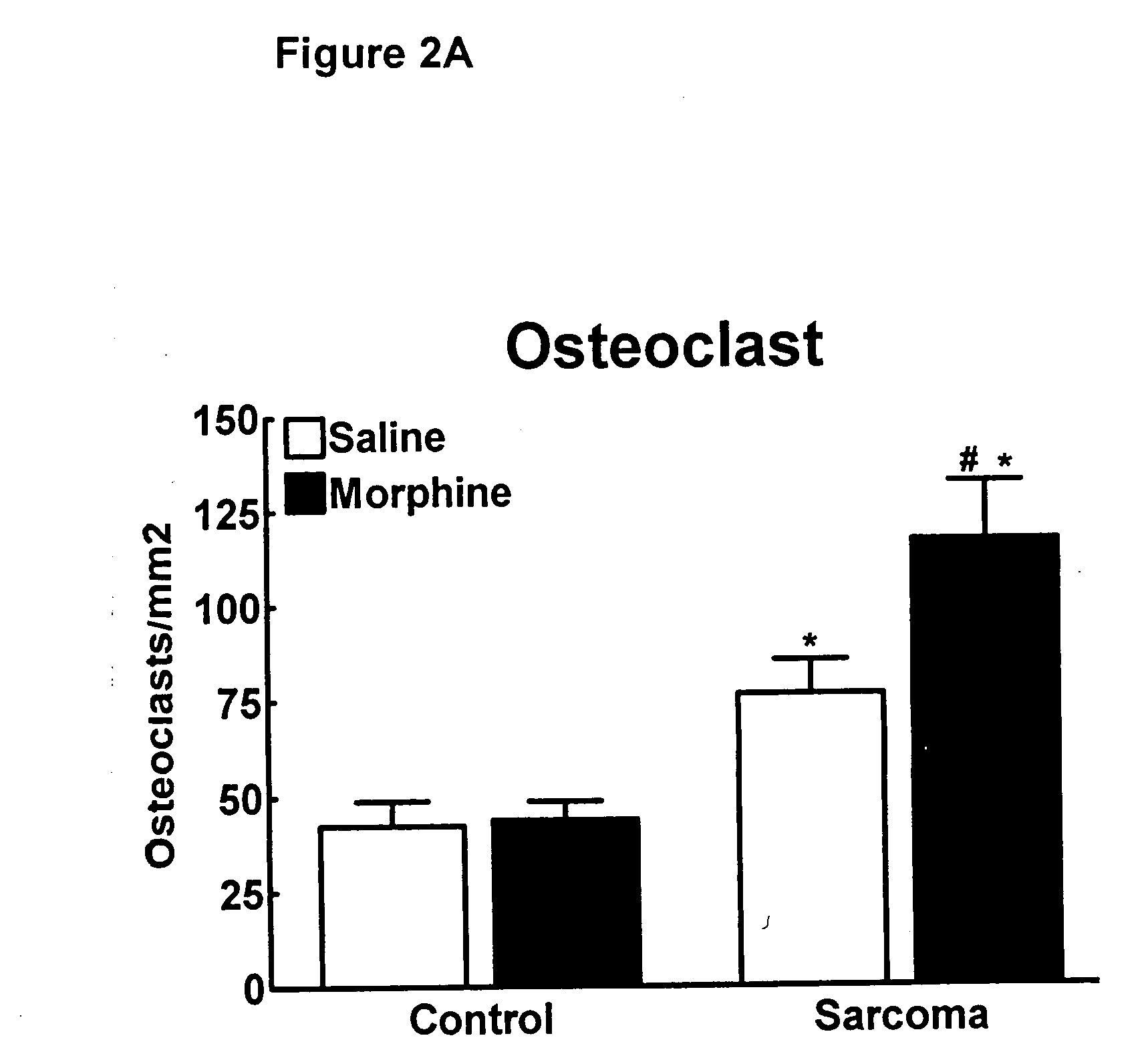
Compositions and methods in the treatment of bone metabolic disorders
InactiveUS20070197573A1Block undesirable peripheral effectAvoid abuseBiocideAnimal repellantsDiseaseOpioid antagonist
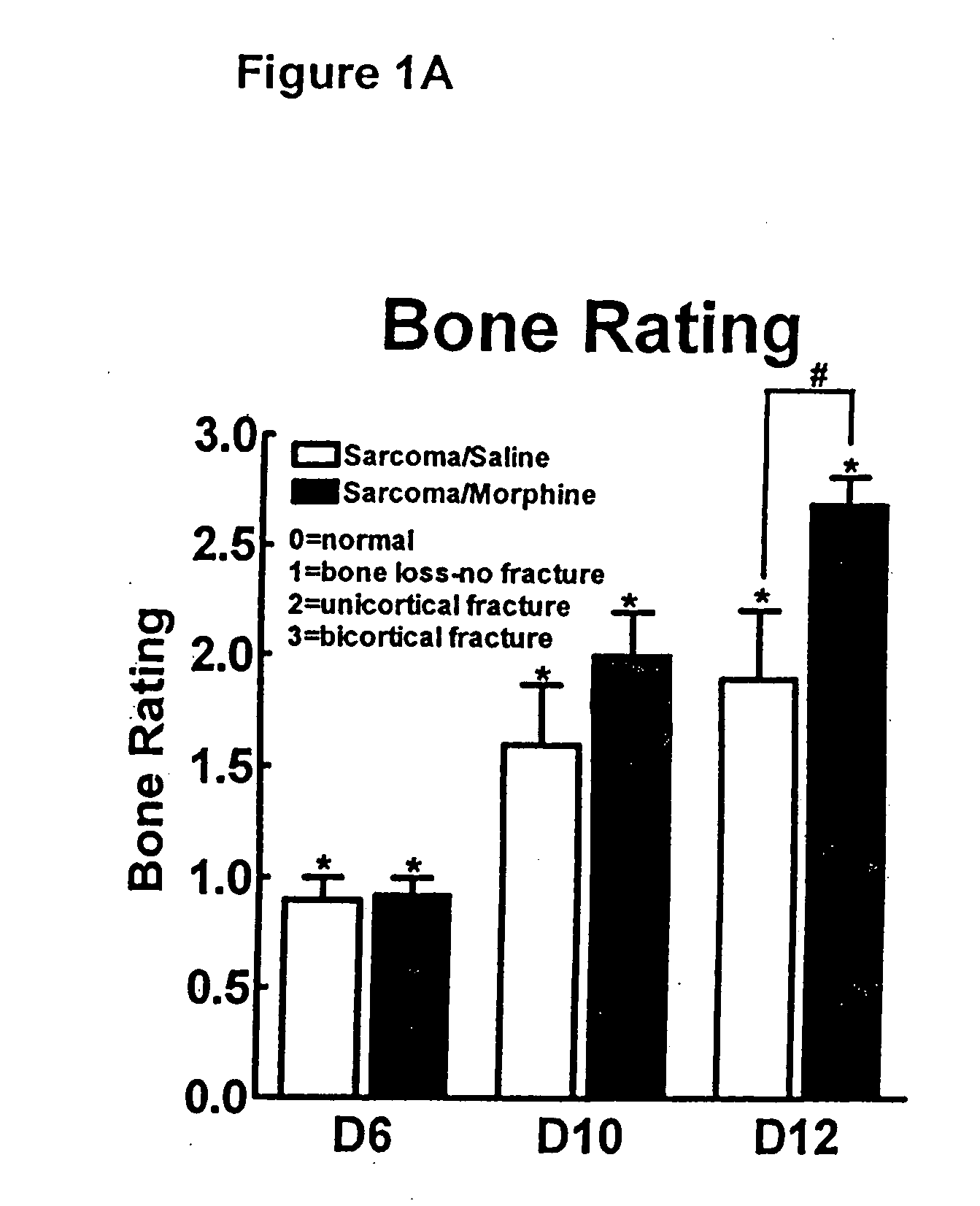
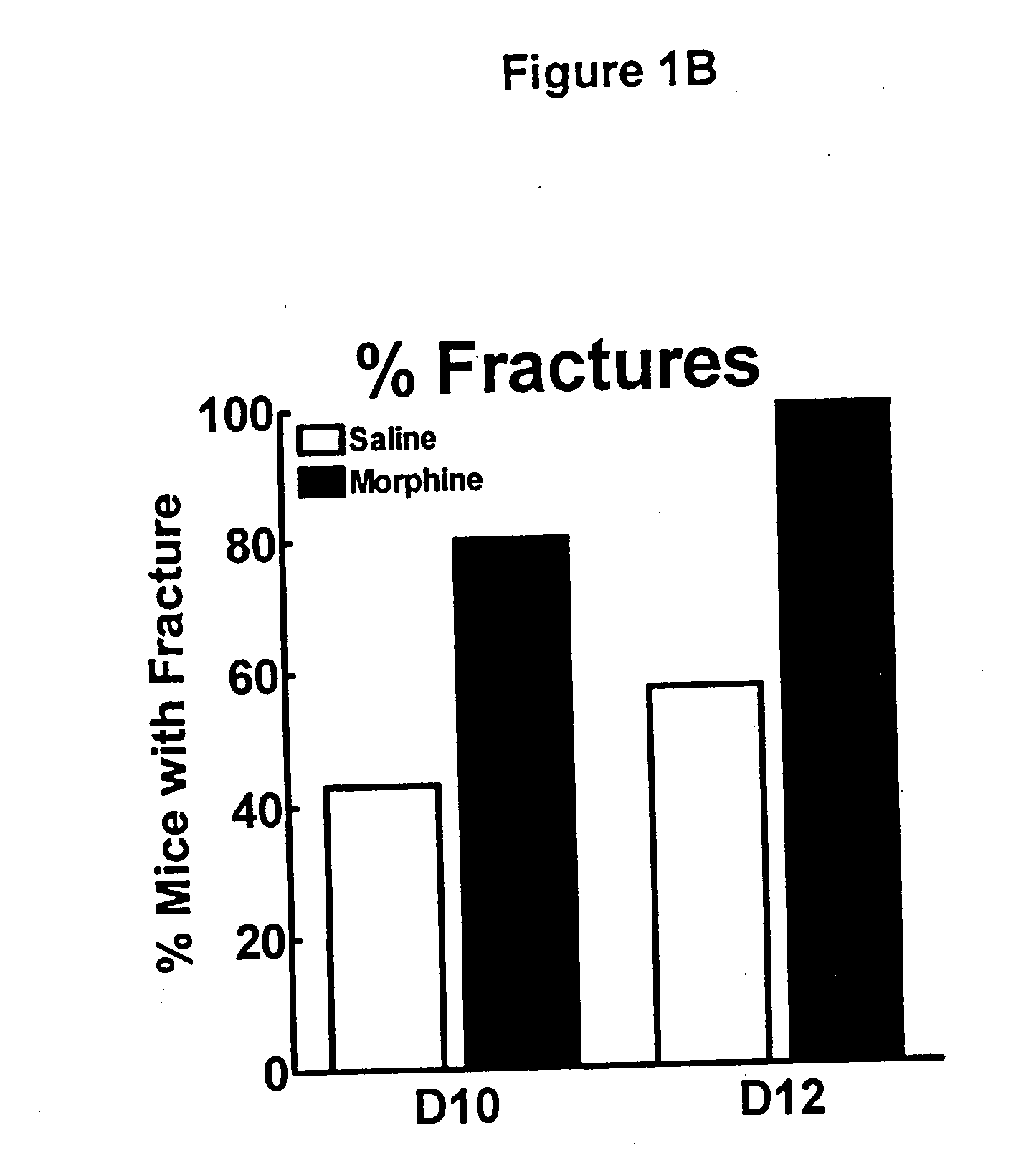
Bone metabolic disorders are treated by administering to an individual a therapeutically effective amount of a peripheral opioid antagonist at one or more of the opioid receptors, including the various naloxone and naltrexone analogs or a pharmaceutically acceptable salt thereof. The invention is further embodied in the use of peripheral antagonists of the opioid receptors, such as the use of naltrexone and naloxone analogs, which can be opioid antagonist with peripheral selectivity at the μ opioid receptor, for the treatment of bone loss, osteoporosis, osteopenia and other bone disorders in individuals using opioid drugs, including patients using opioids for analgesia and in opioid drug-dependent individuals
Owner:AIKO BIOTECH
Dosage form containing oxycodone and naloxone
InactiveUS20110172259A1Quick effectEliminate the effects ofBiocideNervous disorderIn vivoBowel function
Owner:PURDUE PHARMA LP
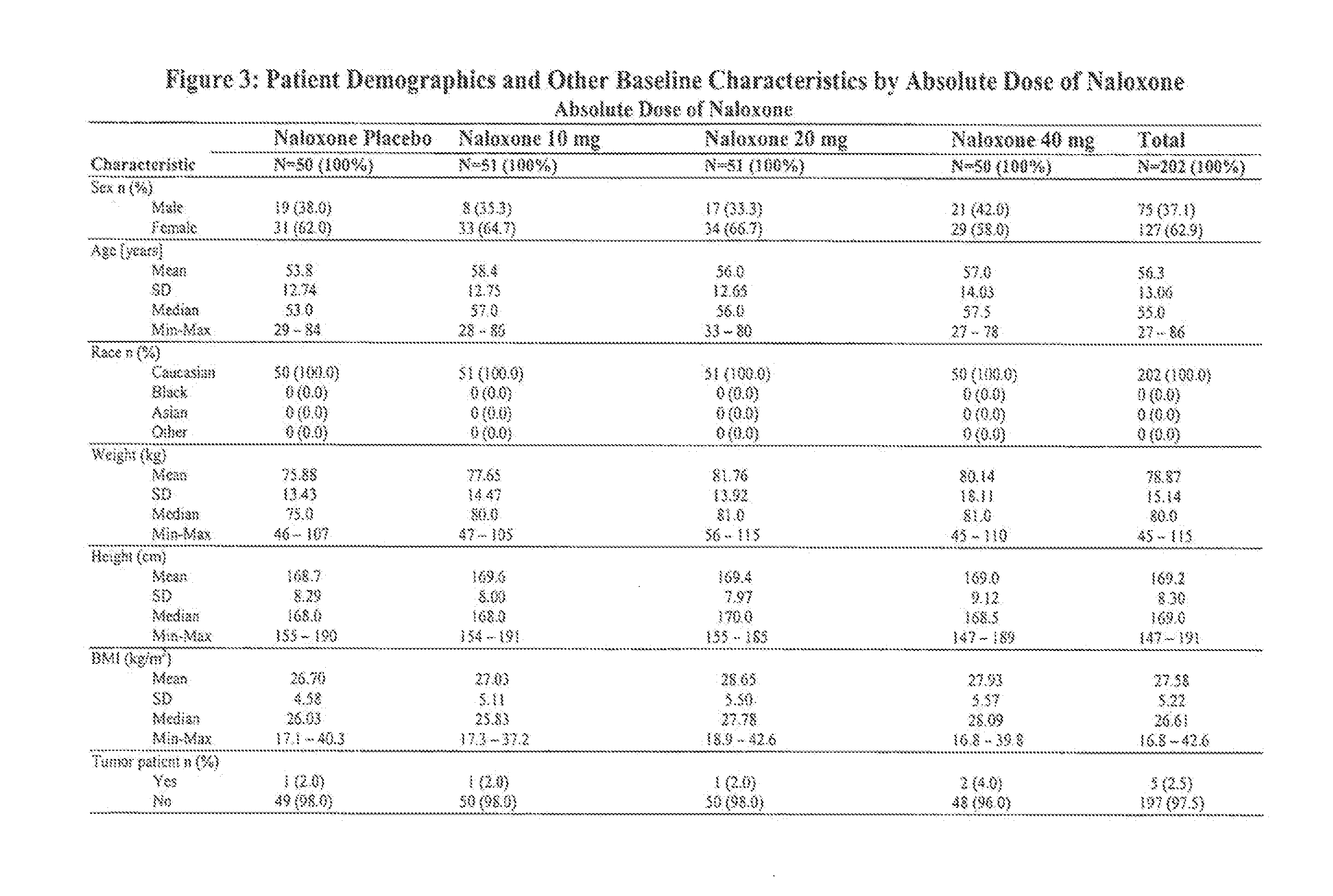
Intranasal naloxone compositions and methods of making and using same
Disclosed herein are compositions containing an opioid antagonist such as naloxone and one or more pharmaceutically acceptable excipients. The compositions may be used for intranasal delivery of Naloxone for the treatment of, for example, opioid overdose in an individual in need thereof. Also disclosed are methods of making compositions containing Naloxone, and devices for nasal delivery of naloxone compositions.
Owner:INDIVIOR UK
Medicament delivery device for administration of opioid antagonists including formulations for naloxone
ActiveUS20140052069A1Prevent undesired leachingMedical devicesMedical syringesOpioid antagonistPharmaceutical drug
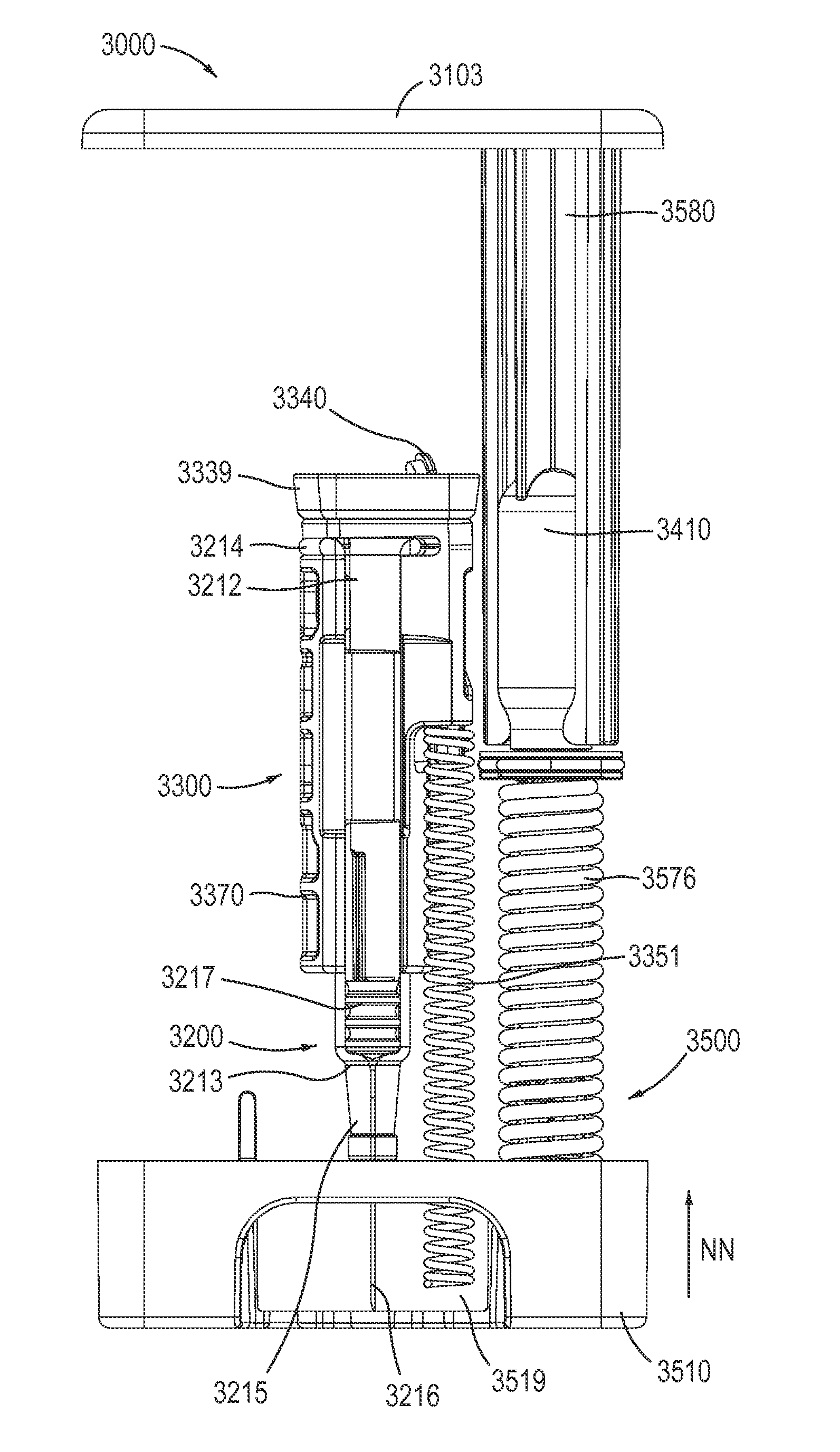
Medicament delivery devices for administration of opioid antagonists are described herein. In some embodiments, an apparatus includes a housing, a medicament container disposed within the housing and an energy storage member disposed within the housing. The medicament container is filled with a naloxone composition that includes naloxone or salts thereof, a tonicity-adjusting agent, and a pH-adjusting agent, whereby the osmolality of the naloxone composition ranges from about 250-350 mOsm and the pH ranges from about 3-5. The energy storage member is configured to produce a force to deliver the naloxone composition.
Owner:KALEO INC
Naloxone hydrchloride freeze-dried powder preparation for injection
InactiveCN1615867AImprove solubilitySimple manufacturing processPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLAlcohol
The freeze dried naloxone hydrochloride powder for injection consists of naloxone hydrochloride in effective medicine amount and proper amount of medicinal carrier. The naloxone hydrochloride content is 0.08-70.59 wt% or 0.1-12 mg; and the medicinal carrier may be one or several of mannitol, glucol, sodium chloride, beta-cyclodextrin, dextran, fructose, sorbic alcohol, etc., is preferably mannitol and glucol and has the ratio in the preparation of 29.41-99.92 wt%.
Owner:YANGGUANG RUNHE SCI & TECHNOLGY BEIJING
Accelerated opiate dependence detoxification process
The present invention provides accelerated detoxification methods for the treatment of a substance abuse-related condition in a subject. A method of the present invention may comprise administering to the subject an effective amount of at least one sedative (e.g, clonidine, diazepam, or olanzapine) and a micro-dose of an opioid antagonist (e.g., naltrexone or naloxone) for at least one day; optionally administering a small dose of an opiate, and administering to the subject a detoxifying amount of a second opioid antagonist (e.g., naloxone); and may further comprise administering to the subject a third opioid antagonist (such as, naltrexone) for an extended period of time (e.g., 12 months).
Owner:COLEMAN PETER R
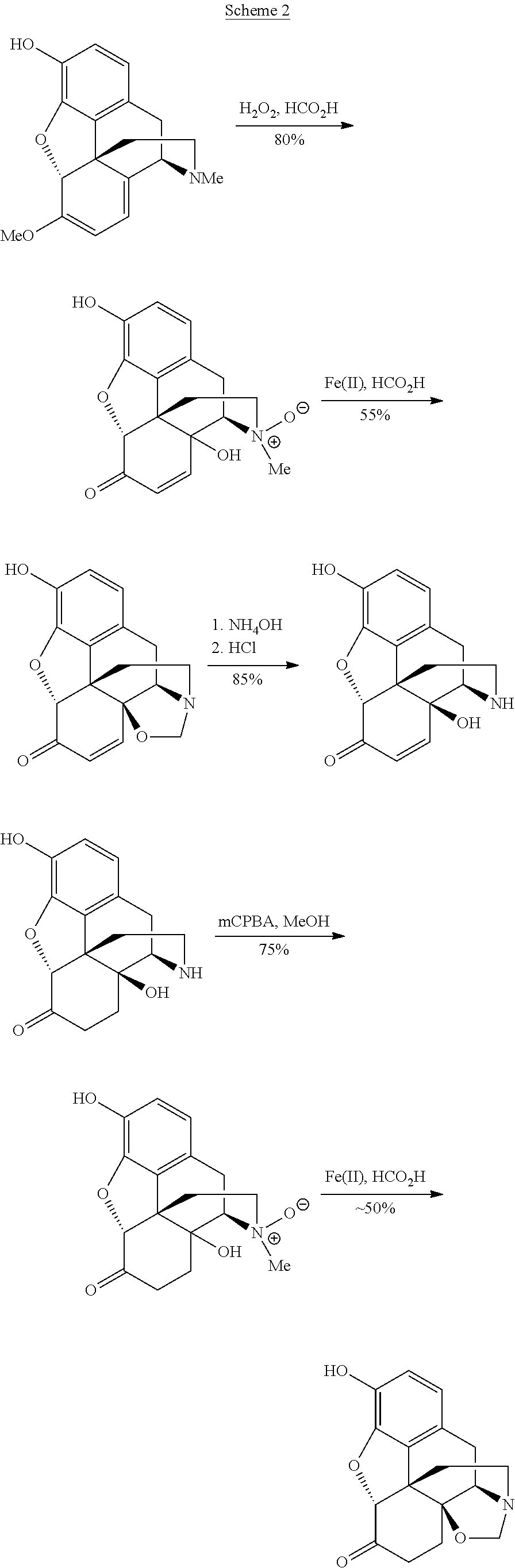
Processes and Intermediates in the Preparation of Morphine Analogs via N-Demethylation of N-Oxides Using Cyclodehydration Reagents
InactiveUS20120283443A1Efficient conversionOrganic chemistryBulk chemical productionMorphinansOxymorphone
A high-yielding method for the N-demethylation of oxycodone- and oxymorphone-N-oxides by the reaction of these compounds with cyclodehydration reagents has been performed. This method has been utilized to improve the synthesis of various morphine analogs, such as naltrexone, nalbuphone and naloxone.
Owner:BROCK UNIVERSITY
Opiates painkiller and opiate receptor antagonist-containing medicinal composition
ActiveCN102068697AGood analgesic effectPrevent and/or mitigate adverse effectsOrganic active ingredientsNervous disorderSide effectNK1 receptor antagonist
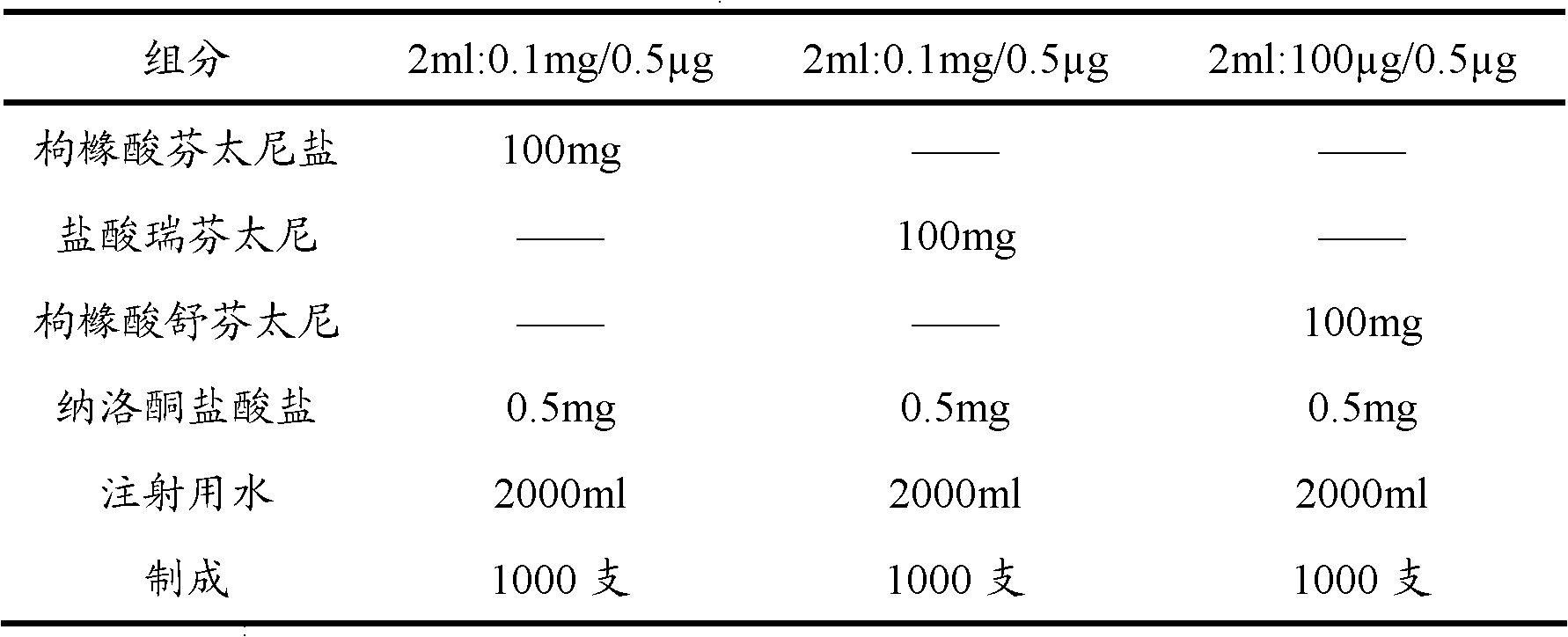
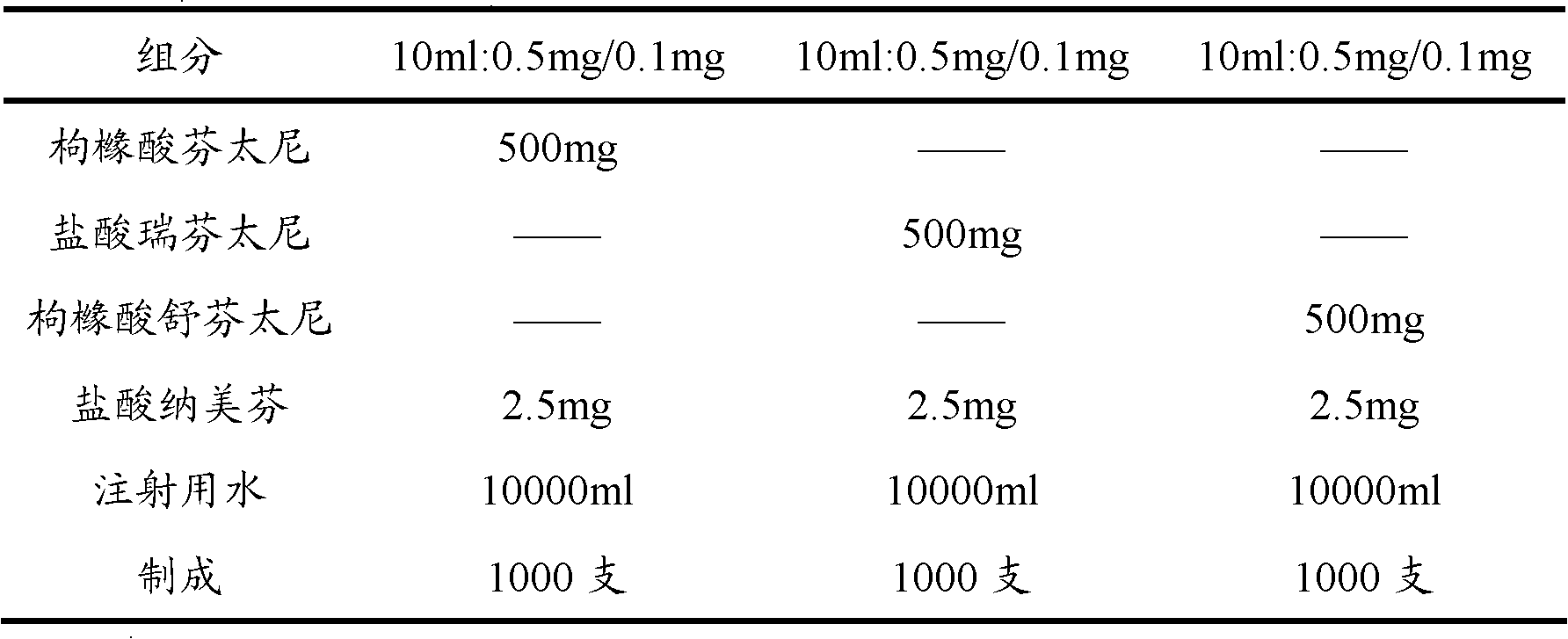
The invention provides an opiates painkiller and opiate receptor antagonist-containing medicinal composition. In the medicinal composition, an opiates painkiller is fentanyl, remifentanil, sufentanil, alfentanil and pharmaceutically acceptable salts thereof; and an opiate receptor antagonist is naloxone, naltrexone, nalmefene and pharmaceutically acceptable salts thereof. The medicinal composition has a pharmacological effect on analgesia. Compared with using the opiates painkiller singly, the composition can prevent and / or lighten side effects in pain treatment, reduce abuse and improve adaptability, and has a reinforcing effect on an analgesic effect of the opiates painkiller.
Owner:YICHANG HUMANWELL PHARMA
Naloxone hydrochloride freeze-dried powder injection and preparation method thereof
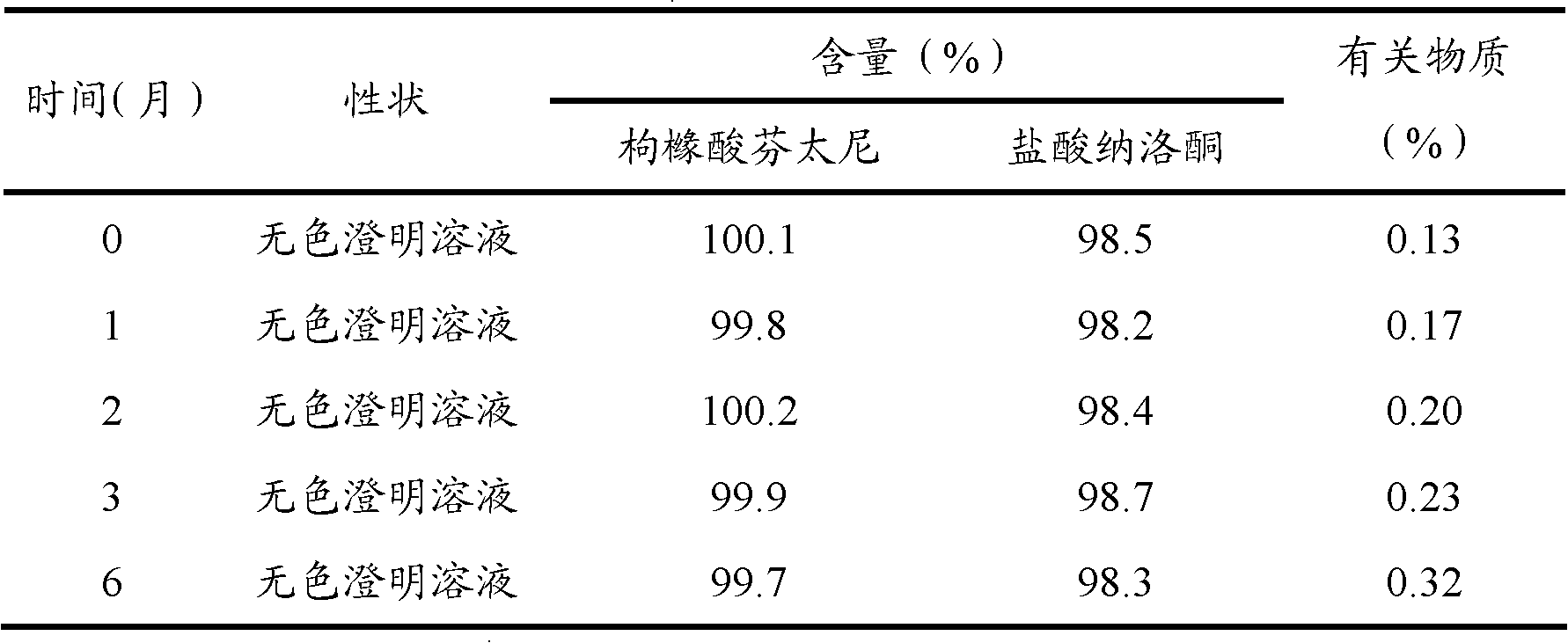
ActiveCN102274196AQuality improvementImprove stabilityOrganic active ingredientsPowder deliveryFreeze-dryingDisodium Edetate
The invention relates to a naloxone hydrochloride freeze-dried powder injection and a preparation method thereof. The naloxone hydrochloride freeze-dried powder injection in every 1000 bottles is obtained by adding water for injection to dissolve the following ingredients and then freeze-drying: 0.1-5g of naloxone hydrochloride, 30-60g of mannitol and 0.01-0.5g of edetate disodium; before the freeze-drying, the pH value of the solution is 2.0 to 5.0. According to the invention, through controlling different pH value regulation ranges during the preparation process of different specifications of the naloxone hydrochloride for injection, the finished products can meet the prescribed standards after the freeze-drying, so that the quality and the stability of the product are improved.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD
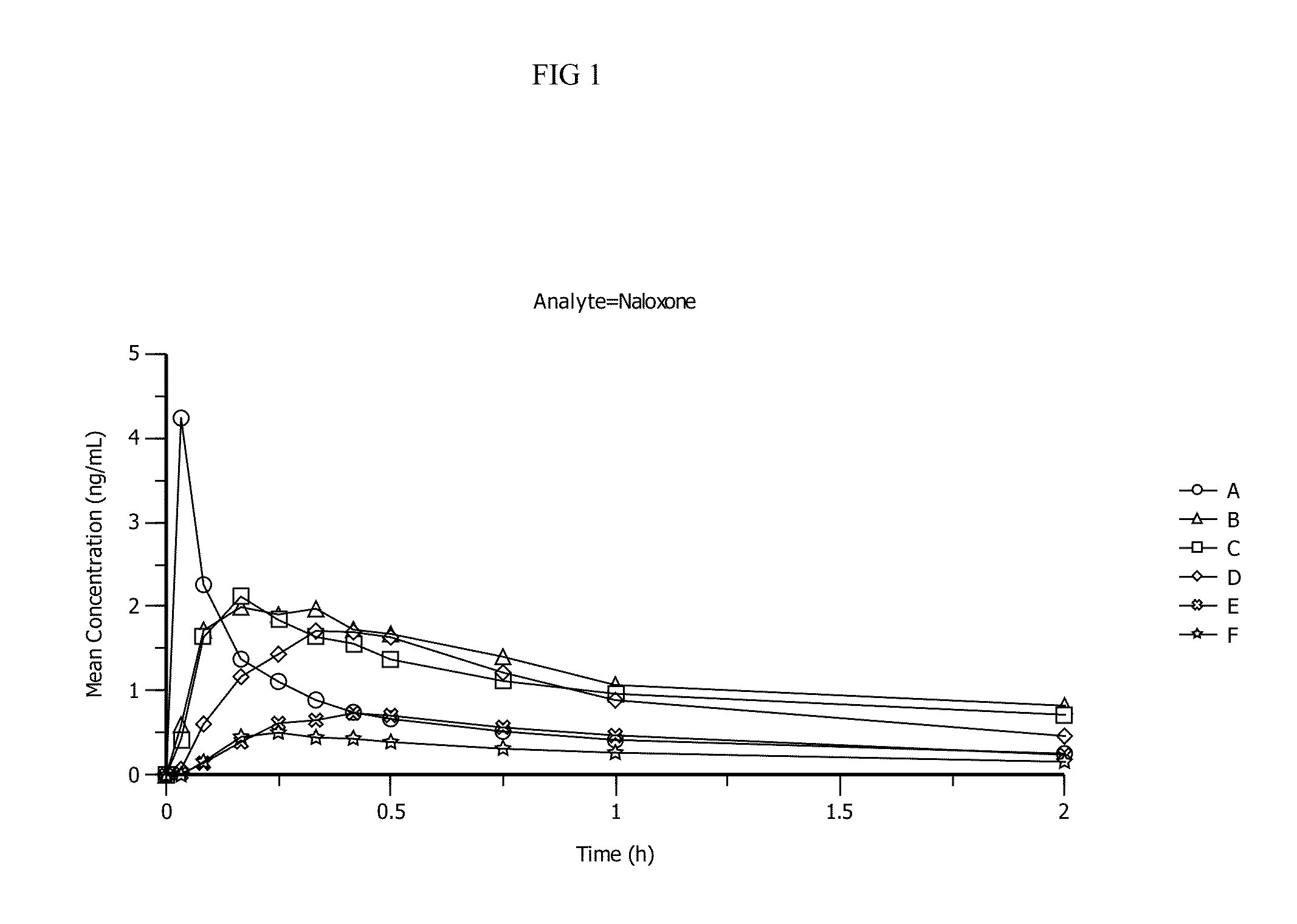
Liquid naloxone spray
ActiveUS10617686B2Organic active ingredientsPharmaceutical delivery mechanismSubstance dependenceOpioid overdose
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention intranasally to a patient in need thereof. Further, the invention provides a method of treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Tricycle compounds and its application in making medicine
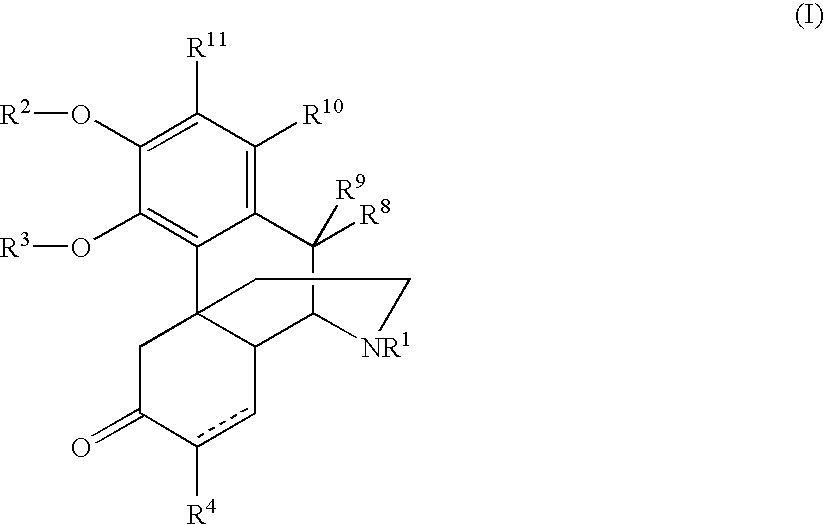
The present invention relates to a tricyclic compound or its stereo-isomer or its pharmaceutically-acceptable salt and their application in preparation of medicine for curing the disease of central nervous system. Besides, said invention also provides the chemical general formula of said tricyclic compound and its detailed description.
Owner:SOUTHEAST UNIV
Abuse potential low compound buprenorphin hydrochloride naloxone hydrochloride sublingual tablet
InactiveCN1943575AReduce Abuse PotentialAvoid abuseOrganic active ingredientsNervous disorderBuprenorphine HydrochlorideAlcohol
A buprenorphine hydrochloride / naloxone hydrochloride sublingual tablets with low misuse potential , said tablets contain medicinal contents of buprenorphine hydrochloride and naloxone hydrochloride, part by weight thereof is 2:1-6:1, 1) using alcohol to dissolve regulator corrigent and bond of PH of prescription amount for use; 2) grinding main medicine buprenorphine hydrochloride / naloxone hydrochloride, filler and lubricant and sifting out thereof; 3) mixing evenly the prescription amount of buprenorphine hydrochloride / naloxone hydrochloride, filler, bond and disintegrating agent, thereafter adding contents in step 1 to prepare into soft stuff, again making into pellet, drying, sorting out, adding lubricant again, mixing evenly and pressing into tablet.
Owner:岳振江
Liquid naloxone spray
ActiveUS10973814B2Organic active ingredientsPharmaceutical delivery mechanismSubstance dependenceOpioid overdose
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid overdose, opioid dependence, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention intranasally to a patient in need thereof. Further, the invention provides a method of treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Liquid naloxone spray
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid overdose, opioid dependence, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention intranasally to a patient in need thereof. Further, the invention provides a method of treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Liquid naloxone spray
ActiveUS10722510B2Organic active ingredientsInorganic non-active ingredientsSubstance dependenceOpioid overdose
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention both intranasally and sublingually to a patient in need thereof. Further, the invention provides a method of treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally and sublingually the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Process for the preparation of morphine analogs via metal catalyzed n-demethylation/functionalization and intramolecular group transfer
The present application is directed to an efficient conversion of C-14 hydroxylated morphine alkaloids to various morphine analogs, such as naltrexone, naloxone and nalbuphone. One feature of this process is an intramolecular functional group transfer from the C-14 hydroxyl to the N-17 nitrogen atom following a palladium-catalyzed N-demethylation.
Owner:BROCK UNIVERSITY
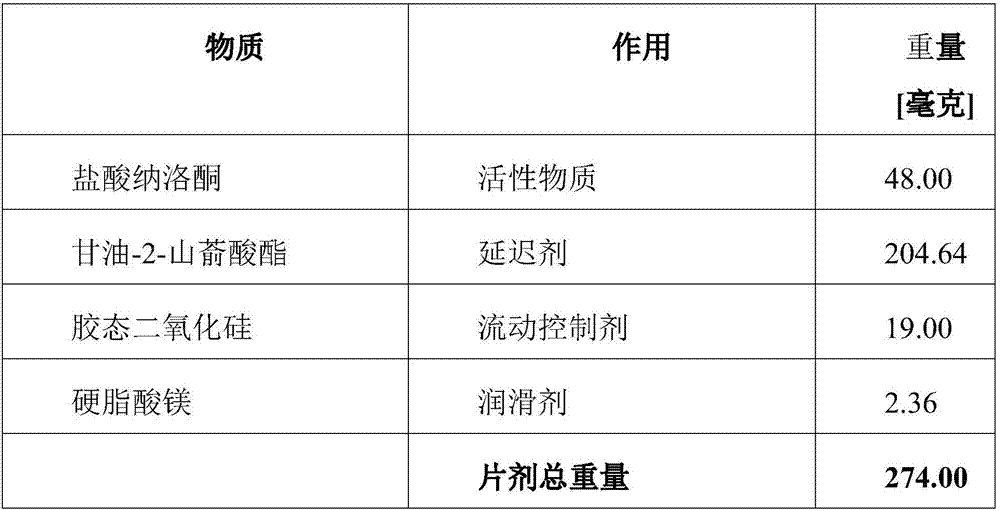
Naloxone monopreparation and multi-layer tablet
InactiveCN107205943AAchieve release kineticsOrganic active ingredientsNervous disorderGlycerolBULK ACTIVE INGREDIENT
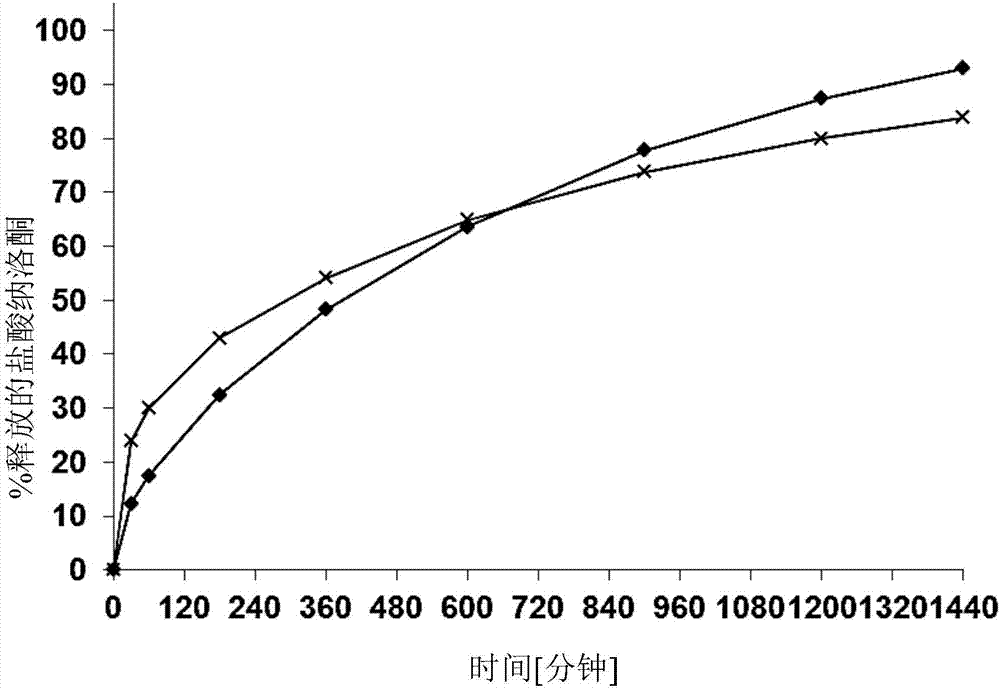
The present invention relates to a solid, oral pharmaceutical composition comprising naloxone, or a pharmaceutically acceptable salt thereof as the active ingredient, the composition having a delayed release of said active ingredient. The composition can comprise a matrix containing glycerol di-behenic acid esters as matrix formers, with a mass ratio of naloxone to matrix former(s) of between 1:1 and 1:10, whereby the active ingredient naloxone has a delayed release. According to the invention, in order to provide a composition suitable for a dosage covering at least twelve hours for treating opioid-induced obstipation, the composition has an in-vitro release rate of the active ingredient, measured using a vane stirrer method according to Eur. Ph. at 75 U / min in 500 ml of 0.1 N hydrochloric acid at 37 DEG C, of 0 % to 75 % in 2 h, 3 % to 95 % in 4 h, 20 % to 100 % in 10 h, 30 % to 100 % in 16 h, 50 % to 100 % in 24 h and more than 80 % in 36 h, said composition having an IC50 / Cmax value of at least 40. Preferably, the composition comprises a value for tmax (naloxone) / tmax (naloxone-3-glucuronoid) of at least 5. In an alternative embodiment, the composition can take the form of a multi-layer tablet.
Owner:德威洛克制药有限公司
Naloxone hydrochloride compound with high purity
The invention relates to a naloxone hydrochloride compound, in particular to a naloxone hydrochloride compound with high purity obtained by a method, belonging to the technical field of medicine. By acid-base reaction, polyamide resin elution and activated carbon absorption, the purity of the naloxone hydrochloride is greatly improved, the product quality of preparation is optimized, and clinic pharmacy safety is ensured; and the method has the advantages of simple process, low cost and high yield, and is suitable for industrialized production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Compounds and methods for treatment of primary biliary cholangitis
The present invention relates to, inter alia, methods of treatment and combinations of (R)-2-(7-(4-cyclopentyl-3-(tri-fluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid (Compound 1) useful for the treatment of primary biliary cholangitis (PBC). In some embodiments, the methods further comprise administering Compound 1, or a pharmaceutically salt, solvate, or hydrate thereof, in combination with a compound selected from the group consisting of: an antihistamine (diphenhydramine), cholestyramine (questran, prevalite), rifampin, an opioid antagonist (naloxone), pilocarpine (isopto carpine, salagen), cevimeline (evoxac), calcium and / or vitamin D supplement, and vitamin A, D, E and / or K supplement. Other embodiments, relate to titration packages for enabling compliance with a regimen of changing dosage of a medication over a period of time for the treatment of primary biliary cholangitis (PBC).
Owner:ARENA PHARMA
Method for inhibiting growth of cancer cells
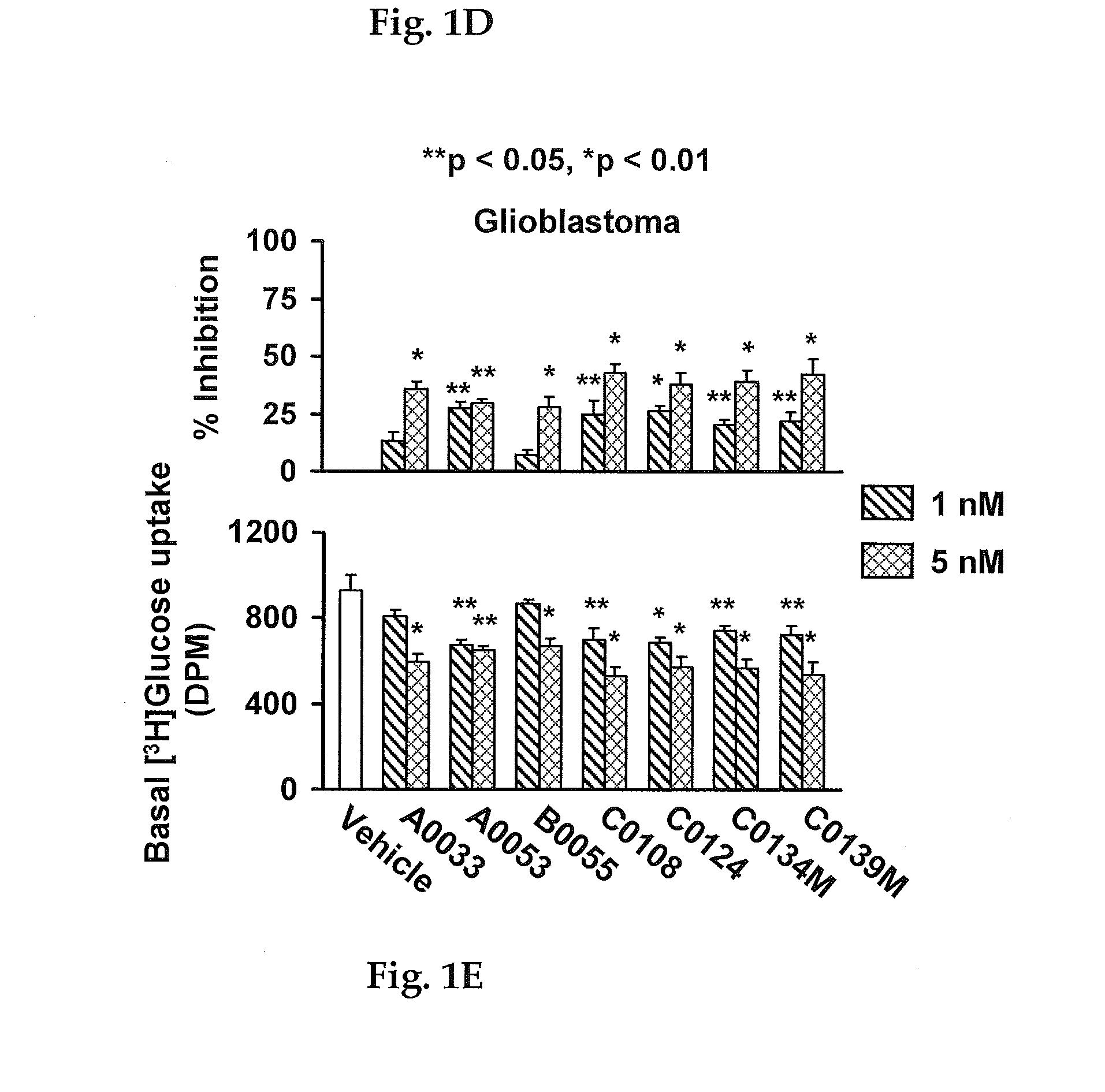
A method of inhibiting the growth of cancer cells is disclosed in which cancer cells that contain an enhanced amount relative to non-cancerous cells of one or more of phosphorylated mTOR, Akt1, ERK2 and serine2152-phosphorylated filamin A are contacted with an FLNA-binding effective amount of a compound or a pharmaceutically acceptable salt thereof that binds to the pentapeptide of filamin A (FLNA) of SEQ ID NO: 1 and exhibits at least about 60 percent of the FITC-labeled naloxone binding amount when present at a 10 μM concentration and using unlabeled naloxone as the control inhibitor at the same concentration. A compound that binds to the FLNA pentapeptide preferably also contains at least four of the six pharmacophores of FIGS. 19-24.
Owner:PAIN THERAPEUTICS INC
Liquid naloxone spray
InactiveCN109922805AOrganic active ingredientsPharmaceutical delivery mechanismOpioidergicOpioid overdose
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt, or a derivative thereof. The invention further provides methods for treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention intranasally to a patient in need thereof. Further, the invention provides a method of treating opioid dependence-, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Compositions and methods for making benzylisoquinoline alkaloids, morphinan alkaloids, thebaine, and derivatives thereof
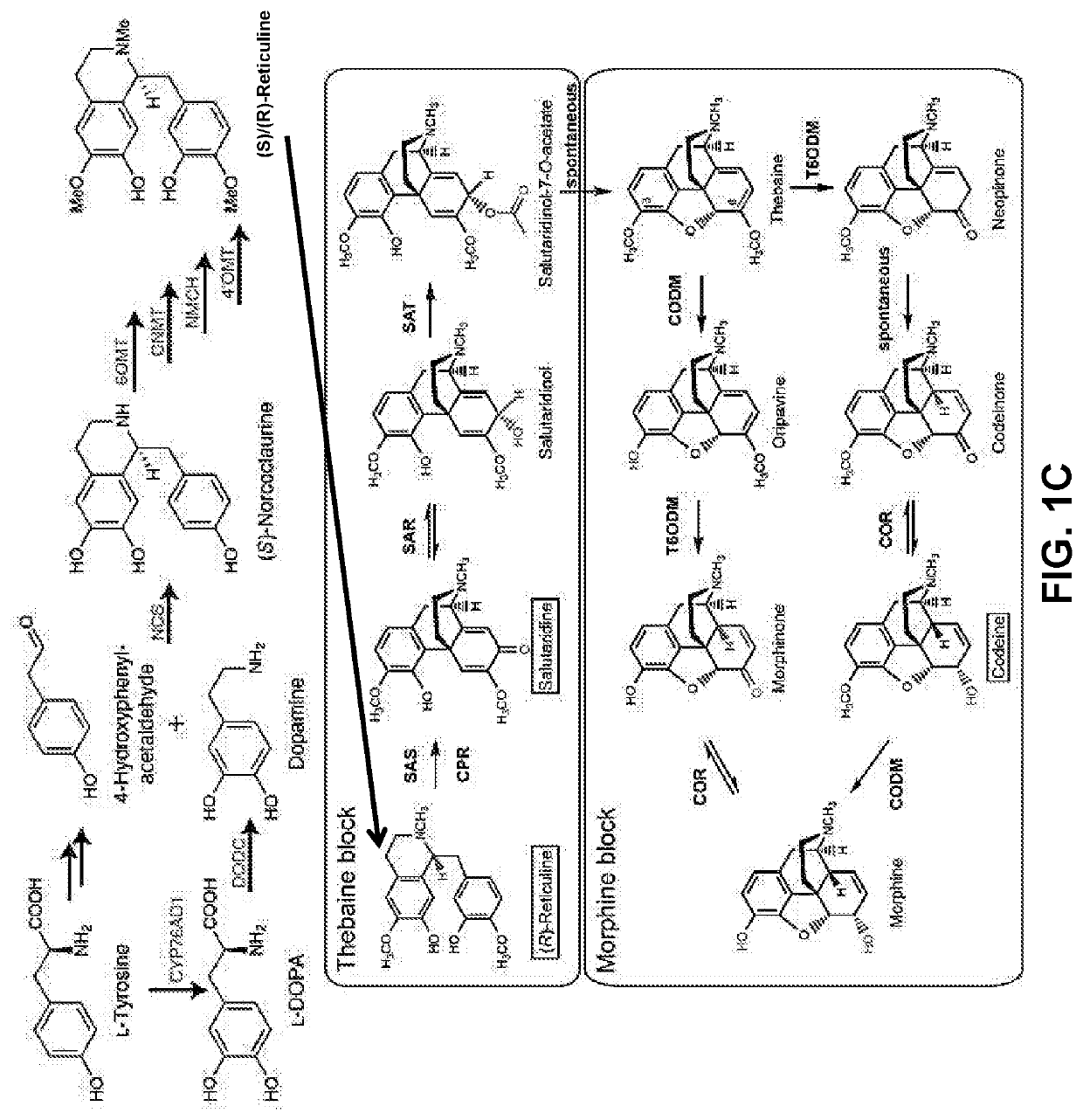
Disclosed herein are methods that may be used for the synthesis of benzylisoquinoline alkaloids (BIAs) such as alkaloid morphinan. The methods disclosed can be used to produce thebaine, oripavine, codeine, morphine, oxycodone, hydrocodone, oxymorphone, hydromorphone, naltrexone, naloxone, hydroxycodeinone, neopinone, and / or buprenorphine. Compositions and organisms useful for the synthesis of BIAs, including thebaine synthesis polypeptides, purine permeases, and polynucleotides encoding the same, are provided.
Owner:ANTHEIA INC
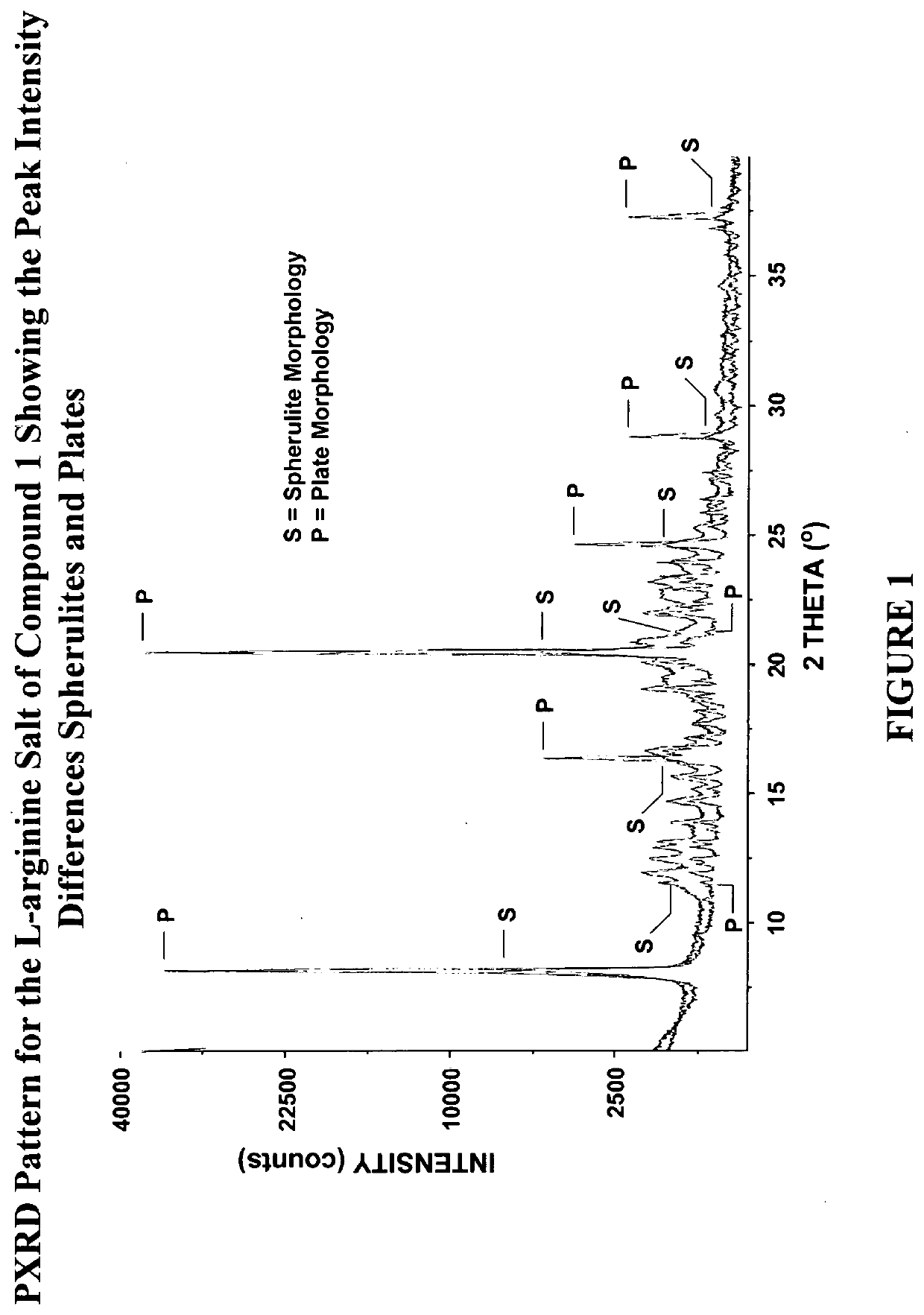
Crystalized polyethylene glycol naloxone oxalate and preparation method
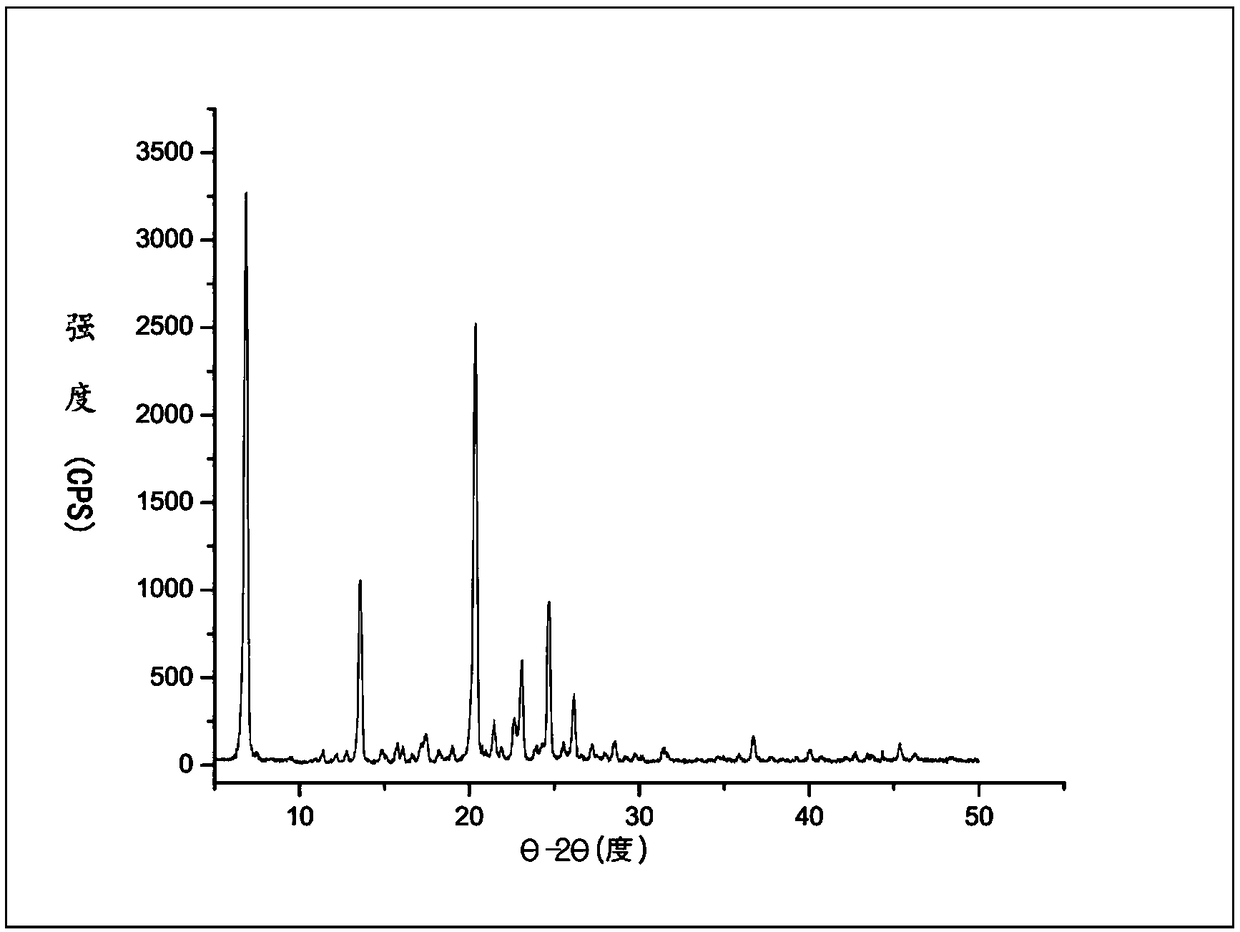
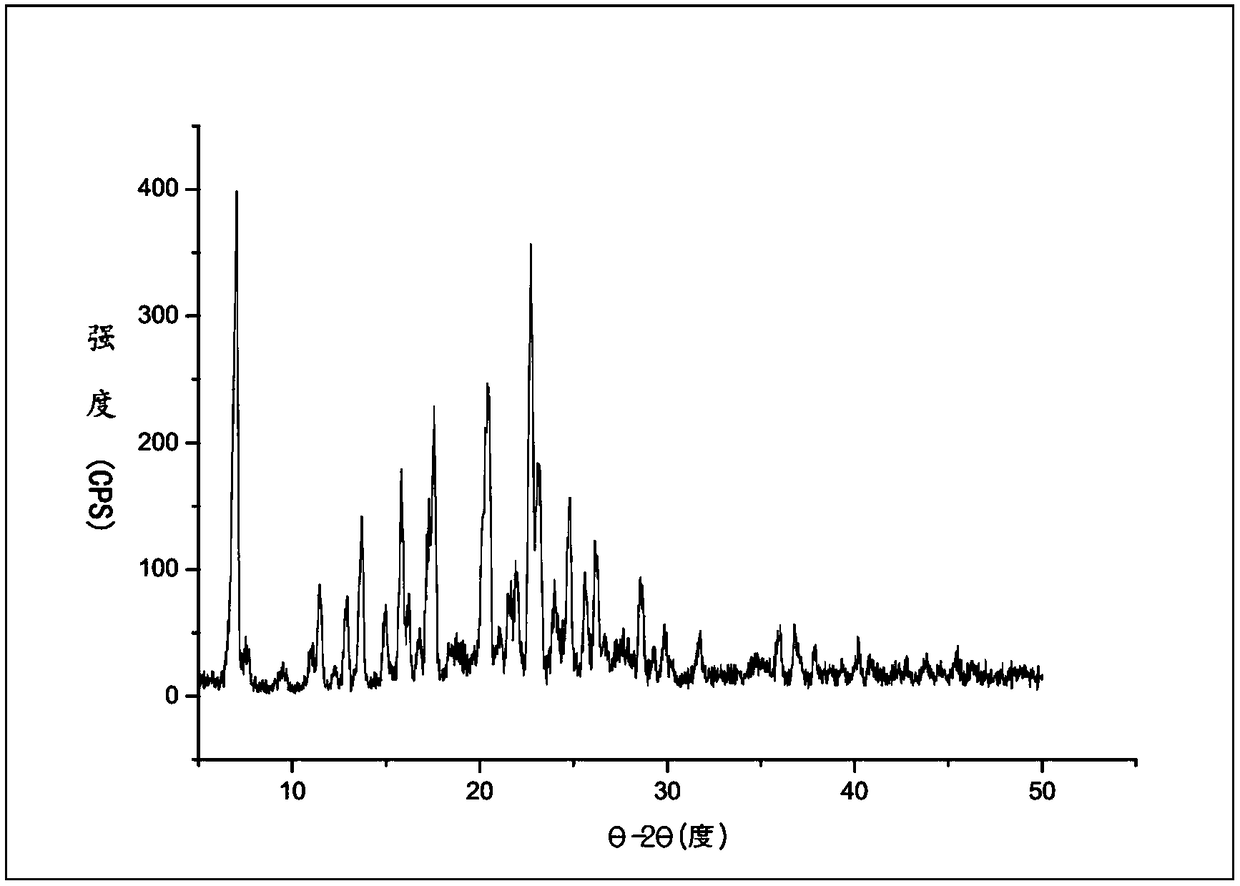
InactiveCN109134479AThe dissolution condition is not highLow reaction temperatureOrganic active ingredientsOrganic chemistry methodsOxalateSolubility
The invention provides novel crystal forms I and II of polyethylene glycol naloxone oxalate. An X-ray powder diffraction map of the two novel crystal forms include diffraction peaks shown by the angleof 2 theta, wherein the error range of the angle of 2 theta ranges from -0.2 to +0.2, the form I includes the peaks of 6.85 degrees, 13.85 degrees, 20.35 degrees, 22.64 degrees, 23.08 degrees, 24.67degrees and 26.15 degrees, and the form II includes the peaks of 7.01 degrees, 11.46 degrees, 12.89 degrees, 13.71 degrees, 14.95 degrees, 15.83 degrees, 17.55 degrees, 20.45 degrees, 21.96 degrees, 22.73 degrees, 25.78 degrees, 25.62 degrees, 26.21 degrees and 28.60 degrees. The invention further provides a preparation method of the novel crystal forms (I and II). The two crystal forms are simplein technology, easy to prepare, low in production cost and environmentally friendly, and has the good water solubility, purity and stability.
Owner:石家庄蒎格医药科技有限公司
Processes and intermediates in the preparation of morphine analogs via N-demethylation of N-oxides using cyclodehydration reagents
InactiveUS8957072B2Efficient conversionOrganic chemistryBulk chemical productionMorphinansOxymorphone
Owner:BROCK UNIVERSITY
Enzymatic preparation of naloxone and pharmaceutical composition thereof
InactiveCN103695497BThe reaction steps are simpleMild reaction conditionsOrganic active ingredientsNervous disorderHuman bodyPharmaceutical drug
The invention provides a method for enzymatic preparation of naloxone. The enzyme is mild in reaction condition and free of harm to a human body. In addition, the invention also provides a naloxone composition prepared by the method and application thereof. The composition contains 14-hydroxy-7,8-dihydro codein ketone impurities, but the medicine performance can not be affected. Furthermore, the invention also provides an identification method of the naloxone composition and enzyme and the like for preparation.
Owner:SUMAAZ CHENGDU BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com