Crystalized polyethylene glycol naloxone oxalate and preparation method
A technology of polyethylene glycol naloxone oxalate and polyethylene glycol naloxone, applied in the field of medicine, can solve the problems of large amount of solvent, long time consumption, low yield, etc., and achieve simplified preparation and post-treatment process , Reduce production costs, improve production efficiency and product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
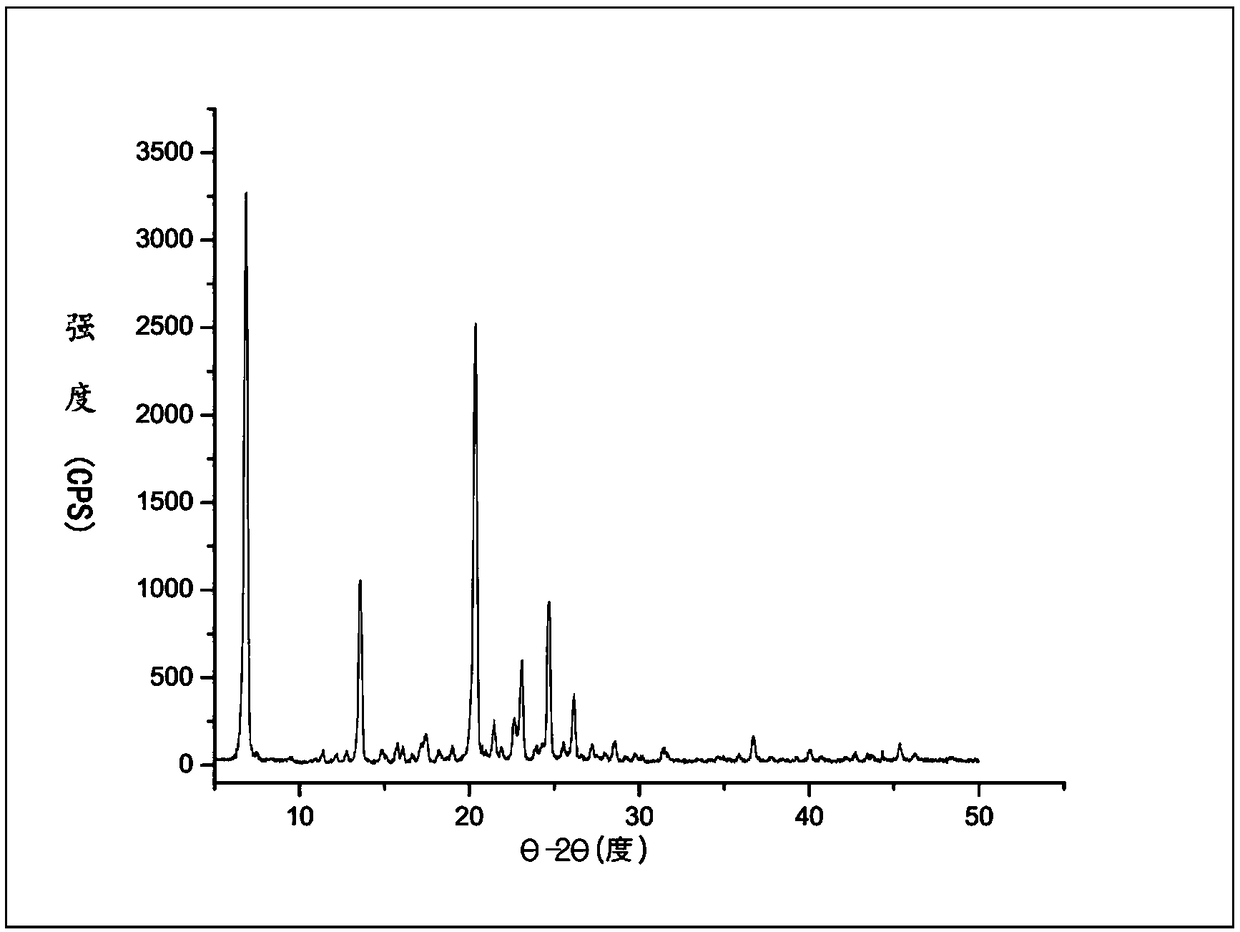
[0029] Example 1, at room temperature (20-35°C), take 30g of polyethylene glycol naloxone free base, add 30mL of methanol and stir to dissolve, then add 5.8g of oxalic acid and stir to dissolve, after 0.5h, dropwise add 300mL of ethyl acetate to crystallize , stirred overnight, filtered with suction, dried under reduced pressure at 40°C for 3h, yield 95%, and obtained material crystal form I, HPLC purity 99.52%, X-ray powder diffraction pattern see figure 1 . The XRPD pattern data for Form I are provided in Table 1.
[0030] Table 1: XRPD values of polyethylene glycol naloxone oxalate crystal form I
[0031]
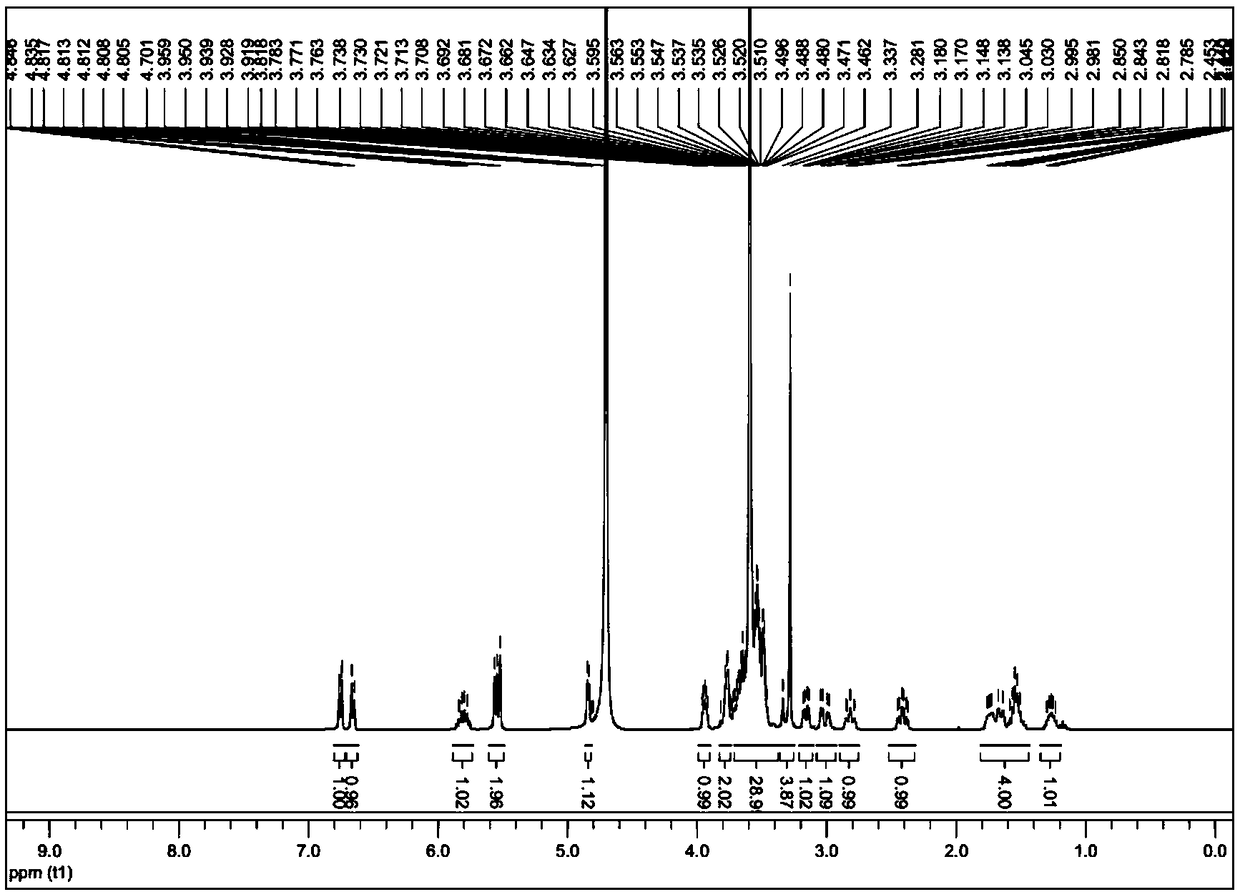
[0032] That 1 H NMR see image 3 ,That 1 H NMR data are as follows:
[0033] 1 H NMR (400MHz, D 2 O)δ: 6.77-6.72 (m, 1H), 6.69-6.63 (m, 1H), 5.87-5.74 (m, 1H), 5.60-5.49 (m, 2H), 4.88-4.82 (m, 1H), 3.97 -3.90(m, 1H), 3.80-3.74(m, 2H), 3.69-3.37(m, 29H), 3.36-3.25(m, 4H), 3.20-3.12(m, 1H), 3.07-2.96(m, 1H), 2.87-2.75(m, 1H), 2.47-2.35(m, 1H), 1.80-1.46(m, 4...
example 2
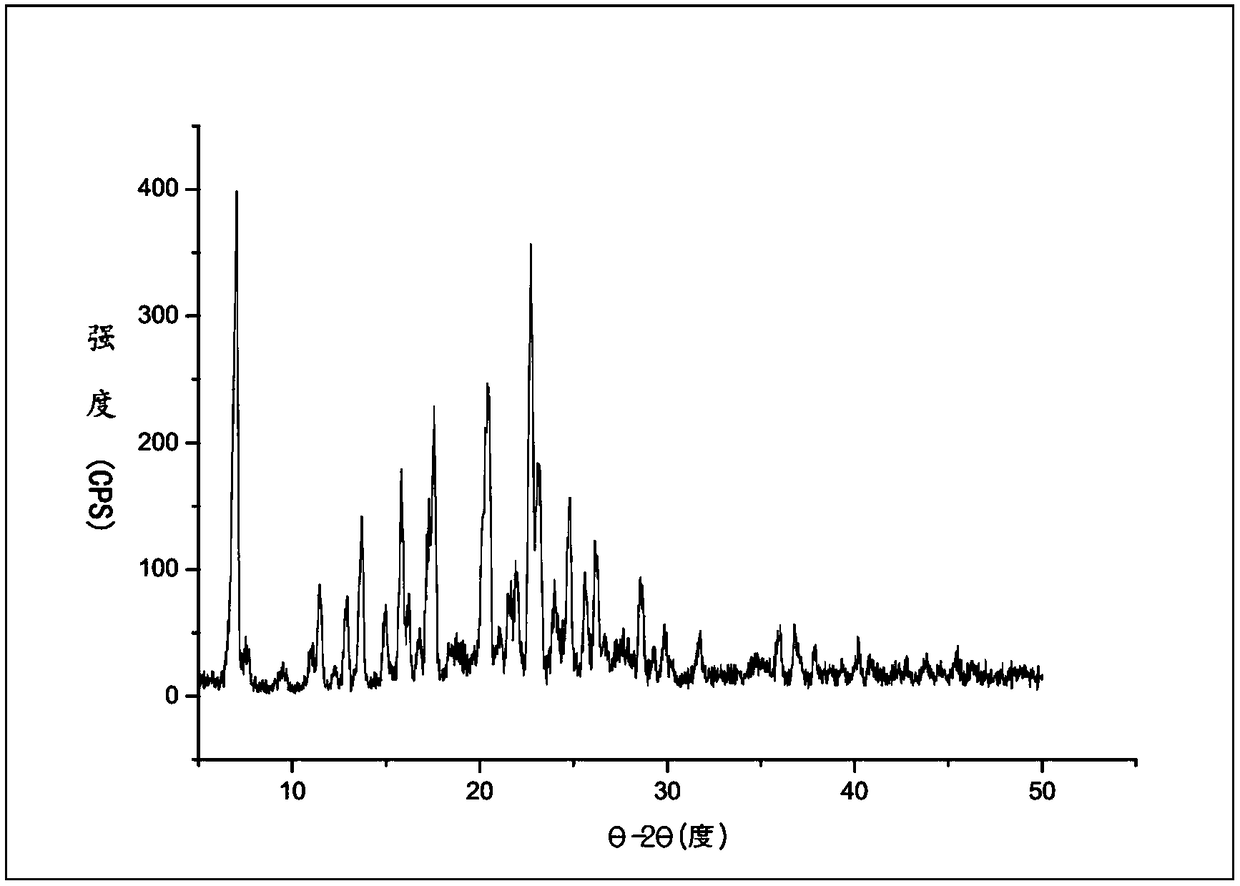
[0036]Example 2, at room temperature (20-35°C), take 30g of polyethylene glycol naloxone free base, add 30mL of methanol and stir to dissolve, then add 5.8g of oxalic acid and stir to dissolve, after 0.5h, dropwise add 300mL of methyl tertiary methyl ether, Crystallize, stir overnight, filter with suction, and dry under reduced pressure at 40°C for 3 hours. The yield is 91%, and the crystal form II is obtained. The HPLC purity is 99.67%. The X-ray powder diffraction pattern is shown in figure 2 .
[0037] The XRPD pattern data for Form II are provided in Table 2.
[0038] Table 2: XRPD value of polyethylene glycol naloxone oxalate crystal form II,
[0039]
[0040]
[0041] That 1 H NMR see Figure 5 ,That 1 H NMR data are as follows:
[0042] 1 H NMR (400MHz, D 2 O)δ: 6.80-6.72 (m, 1H), 6.69-6.63 (m, 1H), 5.87-5.74 (m, 1H), 5.60-5.49 (m, 2H), 4.88-4.82 (m, 1H), 3.97 -3.90(m, 1H), 3.80-3.74(m, 2H), 3.69-3.46(m, 29H), 3.36-3.25(m, 4H), 3.20-3.12(m, 1H), 3.07-2.96...
example 3
[0045] Example 3, take the above-mentioned polyethylene glycol-naloxone oxalate I crystal form 28.5g, microcrystalline cellulose 10.4g, mannitol 20.8g, croscarmellose sodium 1.05g, magnesium stearate 0.35g, and 0.035g of propyl gallate, mixed uniformly according to the method of increasing in equal amounts, and directly compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com