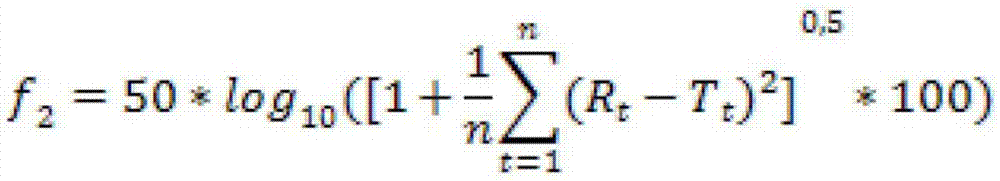Naloxone monopreparation and multi-layer tablet
A multi-layer tablet and naloxone technology, applied in pill delivery, medical preparations of non-active ingredients, digestive system, etc., can solve problems such as impossible to obtain therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
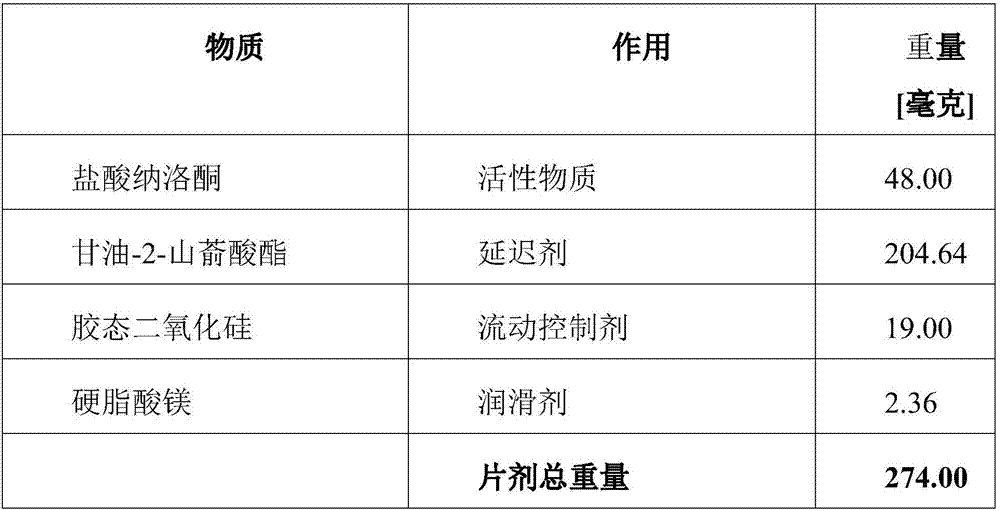
[0121] Tablets of the following compositions are manufactured:
[0122]
[0123] The components naloxone hydrochloride and glycerol-2-behenate were screened and mixed with each other. To obtain the final mixture, the colloidal silicon dioxide in sieved form is mixed first, and then the magnesium stearate. The mixture thus obtained is compressed into tablets by means of a conventional tablet press.
Embodiment 2
[0125] Tablets of the following compositions are manufactured similarly to Example 1:
[0126]
[0127]
[0128] SR consists of 80% by weight polyvinyl acetate, 19% by weight polyvinylpyrrolidone, 0.8% by weight sodium lauryl sulfate and 0.2% by weight colloidal silicon dioxide.
Embodiment 3
[0130] Coated bilayer tablets of the following compositions were manufactured:
[0131]
[0132]
[0133] The component of the naloxone layer, namely naloxone hydrochloride, SR, glyceryl-2-behenate, colloidal silicon dioxide and magnesium stearate were sieved and mixed with each other to form a first powder mixture. The other components of the placebo layer, ie, sugar granules, hypromellose, microcrystalline cellulose, colloidal silicon dioxide, and magnesium stearate, were sieved and mixed with each other to form a second powder mixture.
[0134] The first and second blends are compressed into bilayer tablet cores by means of a conventional bilayer tablet press. The bilayer tablet core thus obtained is Coating is carried out in a coater at a temperature between 30°C and 50°C, so that bilayer tablets are obtained.
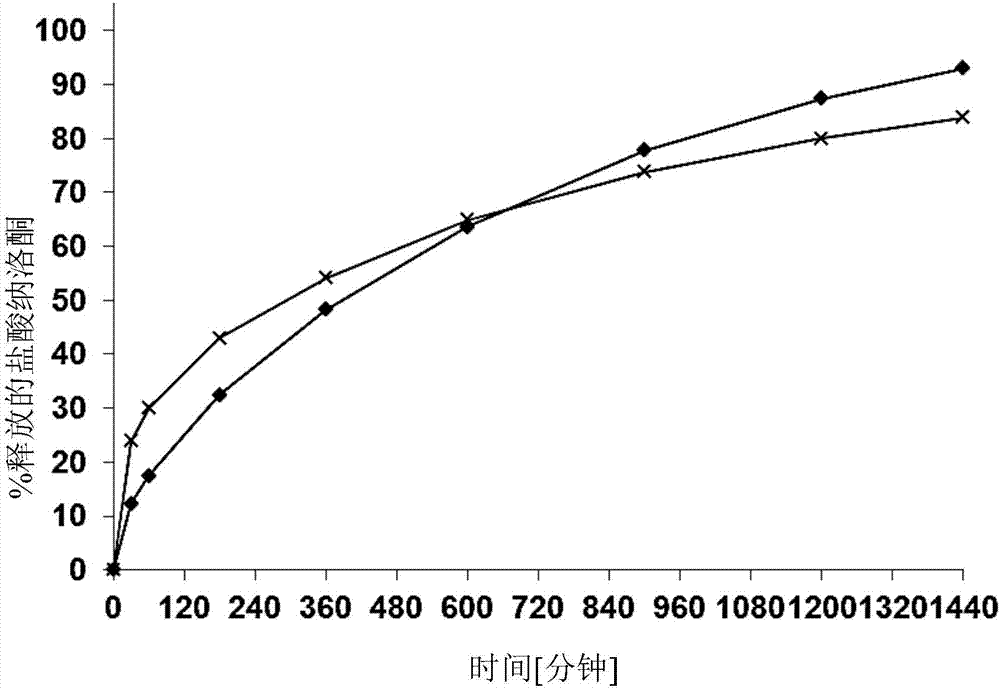
[0135] release curve
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com