Naloxone hydrochloride nano granule powder injection formulation and preparation thereof
A technology of naloxone hydrochloride and nanoparticles, applied in the field of medicine, can solve the problems of quality decline and unstable pH value, and achieve the effects of stable pH value, rapid reconstitution and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
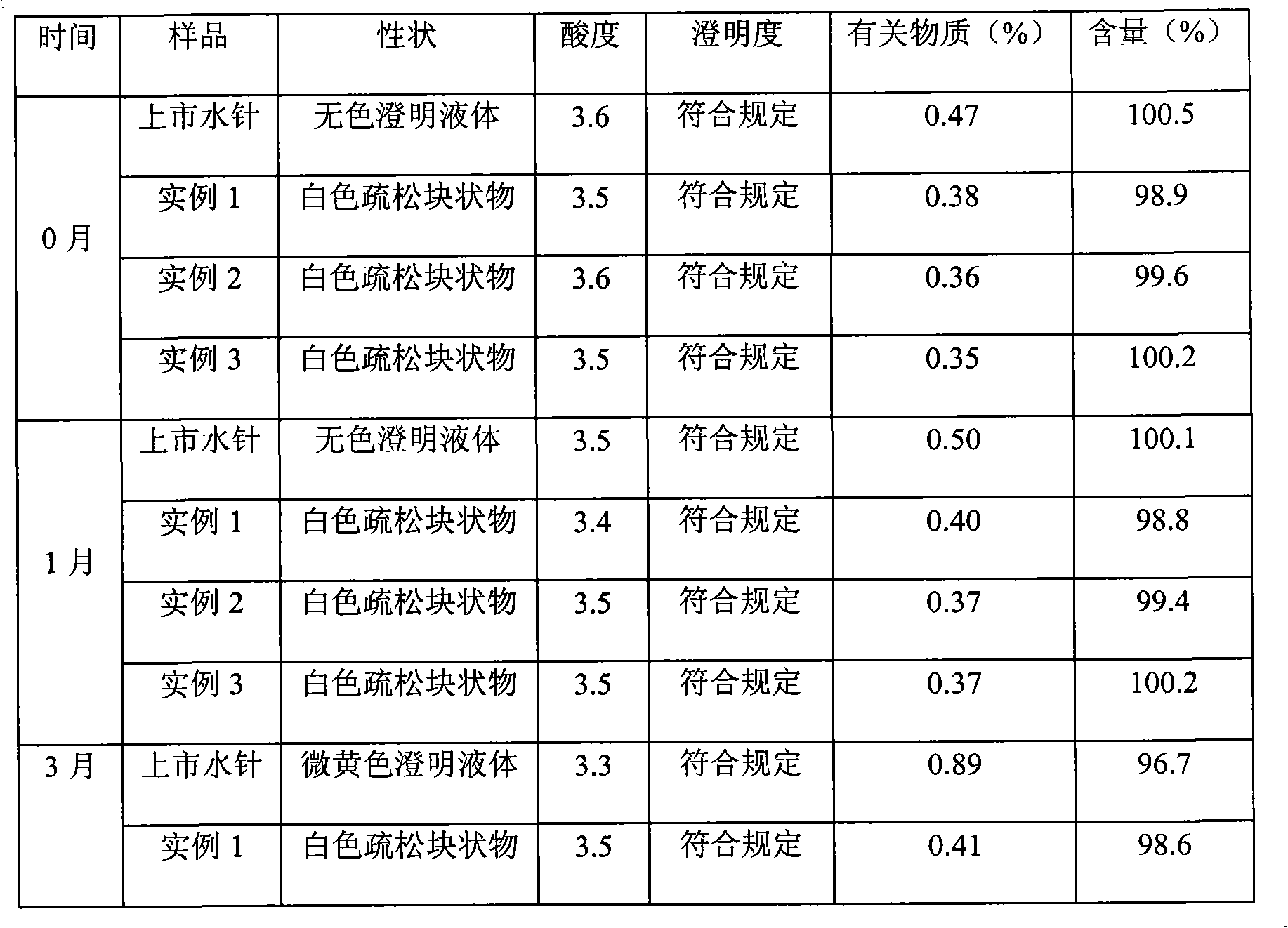
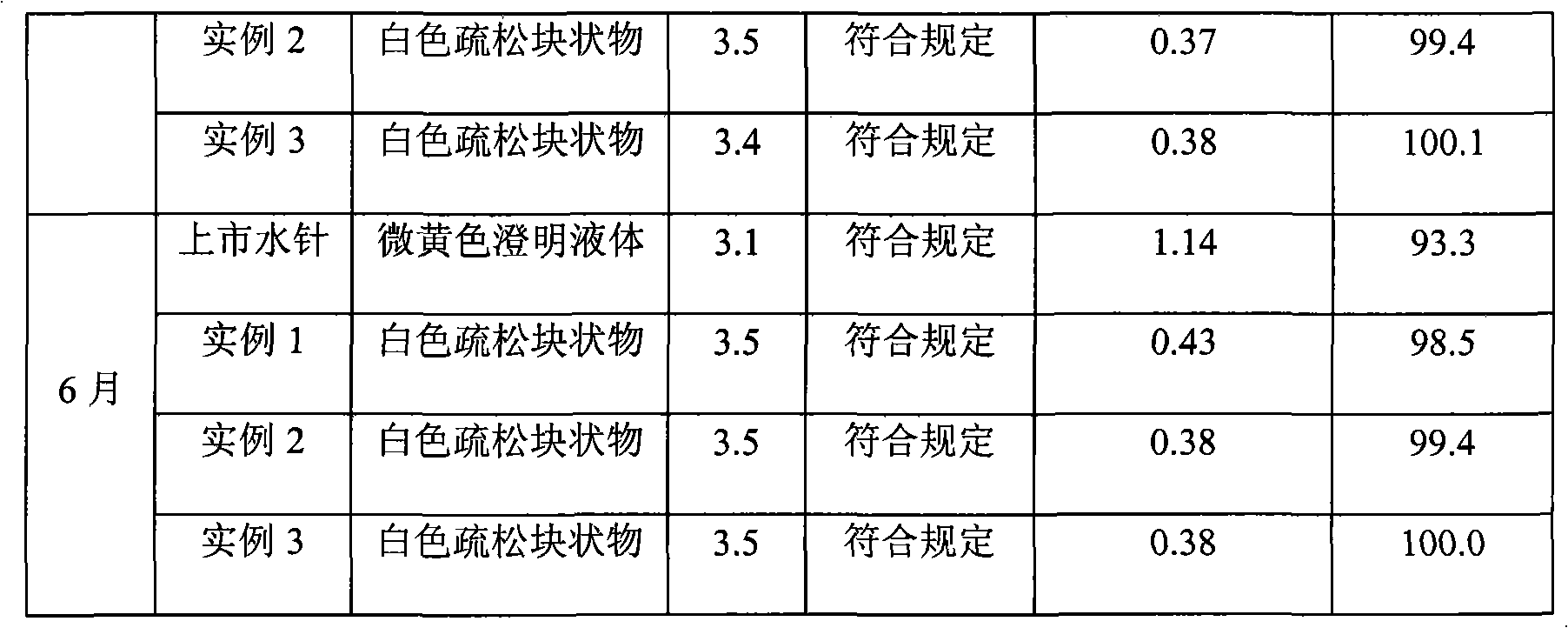
[0030] Example 1: Prescription
[0031] Naloxone Hydrochloride 0.4g
[0032] Dextran 0.15g
[0035] Methyl cyanoacrylate 0.6g
[0036] Mannitol 10g
[0037] Water for injection 1000ml
[0038] Preparation Process:
[0039] 1. Weigh 0.4g of naloxone hydrochloride, 0.15g of dextran and 0.5g of sodium bisulfite, add 200ml of water for injection to dissolve, adjust the pH value to 2.2 with 1mol / L hydrochloric acid solution, and stir evenly;
[0040] 2. Slowly add 0.6 g of methyl cyanoacrylate under electromagnetic stirring, continue stirring at room temperature for 3 hours, add 0.2 g of sodium sulfate, continue stirring for 3 hours, and adjust the pH value to 3.7 with 0.5 mol / L sodium hydroxide solution, Add water for injection to 800ml, stir well;
[0041] 3. Add 10g of mannitol, stir to dissolve, set the volume to 1000ml, add 0.5g of charcoal for needles, stir at room temperature for 10 minutes, filter for decar...
Embodiment 2
[0048] Example 2: Prescription
[0049] Naloxone Hydrochloride 4g
[0050] Dextran 2g
[0051] Sodium bisulfite 8g
[0052] Sodium sulfate 4g
[0053] Ethyl cyanoacrylate 10g
[0054] Glucose 1200g
[0055] Water for injection 4000ml
[0056] Preparation Process:
[0057] 1. Weigh 4g of naloxone hydrochloride, 2g of dextran and 8g of sodium bisulfite, add 2000ml of water for injection to dissolve, adjust the pH value to 2.4 with 1mol / L hydrochloric acid solution, and stir evenly;
[0058] 2. Slowly add 10g of ethyl cyanoacrylate under electromagnetic stirring, continue to stir at room temperature for 3 hours, add 4g of sodium sulfate, and continue to stir for 3 hours, adjust the pH value to 3.6 with 0.5mol / L sodium hydroxide solution, add Water for injection to 3600ml, stir well;
[0059] 3. Add 1200g of glucose, stir to dissolve, set the volume to 4000ml, then add 20g of charcoal for needles, stir at room temperature for 30 minutes, filter for decarburization, and the...
Embodiment 3
[0062] Example 3: Prescription
[0063] Naloxone Hydrochloride 2g
[0064] Dextran 1.2g
[0065] Sodium bisulfite 4.3g
[0066] Sodium sulfate 2.5g
[0067] Propyl cyanoacrylate 5.6g
[0068] Sucrose 400g
[0069] Water for injection 2000ml
[0070] Preparation Process:
[0071] 1. Weigh 2g of naloxone hydrochloride, 1.2g of dextran and 4.3g of sodium bisulfite, add 1000ml of water for injection to dissolve, adjust the pH value to 2.5 with 1mol / L hydrochloric acid solution, and stir evenly;
[0072] 2. Slowly add 5.6g of propyl cyanoacrylate under electromagnetic stirring, continue stirring at room temperature for 3 hours, add 2.5g of sodium sulfate, continue stirring for 3 hours, adjust the pH value to 3.5 with 0.5mol / L sodium hydroxide solution, Add water for injection to 1600ml, stir well;
[0073] 3. Add 400g of sucrose, stir to dissolve, set the volume to 2000ml, then add 5g of charcoal for needles, stir at room temperature for 20 minutes, filter for decarburizati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com