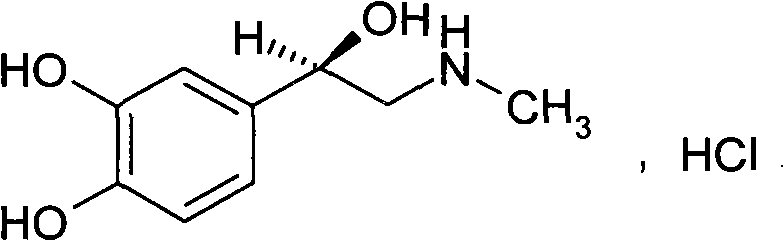
Adrenaline hydrochloride injection and preparation process thereof
A technology of epinephrine hydrochloride and epinephrine, applied in anesthetics, blood diseases, extracellular fluid diseases, etc., can solve problems such as unguaranteed and difficult to meet the quality standards of epinephrine hydrochloride injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0030] (1) Add 90% of the total amount of water for injection below 30°C in the preparation container, and pass CO 2 15 minutes to make it saturated;
[0031] (2) Sodium chloride is dissolved in an appropriate amount of water for injection below 30°C, and the appropriate amount accounts for about 5% of the total preparation amount;
[0032] (3) Dissolve edetate disodium with boiled water for injection;
[0033] (4) Dissolve the sodium chloride solution obtained in step (2), the edetate disodium solution obtained in step (3), sodium metabisulfite and epinephrine in the water for injection obtained in the above step (1), stir to dissolve ;
[0034] (5) Measure the intermediate pH of the solution obtained in step (4), adjust the pH to 3.6-4.0 with 1mol / L hydrochloric acid, add water for injection until the total amount is stirred;
[0035] (6) filter the solution obtained in step (5) with a filter membrane of 0.45um and 0.22μm;
[0036] (7) The solution obtained in step (6) i...
preparation Embodiment 2
[0038] (1) Add 900g of water for injection below 30°C to the preparation container, pass through CO 2 15 minutes to make it saturated;
[0039] (2) Dissolve 7.944g of sodium chloride in 50g of water for injection below 30°C;
[0040] (3) Dissolve edetate disodium 0.298g with 10g of boiled water for injection;
[0041] (4) The sodium chloride solution obtained in step (2), the edetate disodium solution obtained in step (3), 0.993 g of sodium metabisulfite and 0.993 g of adrenaline were dissolved in the water for injection obtained in the above step (1) , stir to dissolve;
[0042] (5) Measure the pH of the solution obtained in step (4), adjust the pH to 3.6-4.0 with 1mol / L hydrochloric acid, add water for injection to 1000g and stir well;
[0043] (6) filter the solution obtained in step (5) with a filter membrane of 0.45um and 0.22μm;
[0044] (7) The solution obtained in step (6) is subjected to aseptic potting according to the potting operation procedure, and N is connec...
preparation Embodiment 3
[0046] (1) Add 1800g of water for injection below 30°C to the preparation container, pass through CO 2 15 minutes to make it saturated;
[0047] (2) Dissolve 15.888g of sodium chloride in 100g of water for injection below 30°C;
[0048] (3) Dissolve edetate disodium 0.596g with 20g of boiled water for injection;
[0049] (4) Dissolve the sodium chloride solution obtained in step (2), the edetate disodium solution obtained in step (3), sodium metabisulfite 1.986g and epinephrine 1.986g in the water for injection obtained in the above step (1) , stir to dissolve;
[0050] (5) Measure the pH of the solution obtained in step (4), adjust the pH to 3.6-4.0 with 1mol / L hydrochloric acid, add water for injection to 2000g and stir well;
[0051] (6) filter the solution obtained in step (5) with a filter membrane of 0.45um and 0.22μm;
[0052] (7) The solution obtained in step (6) is subjected to aseptic potting according to the potting operation procedure, and N is connected in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com