Famotidine injection and preparation method thereof
A famotidine and injection technology, which is applied in the field of biomedicine, can solve the problems of cumbersome inspection operations, unsuitable semi-finished products, and many losses, and achieves the effects of ensuring product quality, improving yield and considerable benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
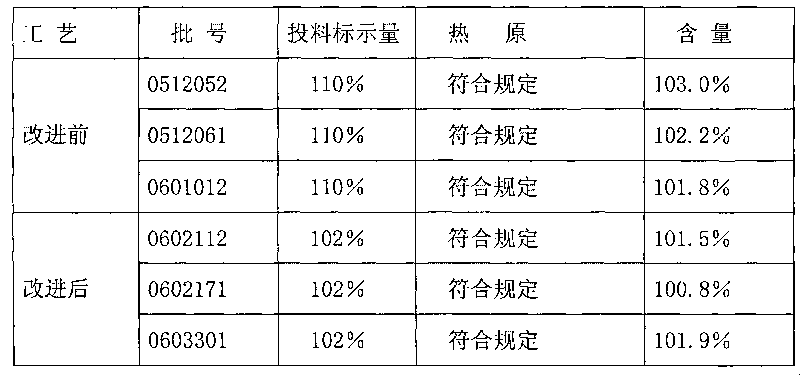
[0057] Example: 1000ml of famotidine injection contains the following raw materials: 20.0g of famotidine, 3.5g of aspartic acid, 0.2g of edetate disodium, 0.5g of medicinal charcoal, and the balance is water for injection.
[0058] The preparation method of above-mentioned famotidine injection, its concrete steps are as follows:
[0059] ①. Take 80% fresh water for injection, add the formulated amount of aspartic acid and disodium edetate, and stir until completely dissolved;
[0060] ②. Add medicinal charcoal in the prescribed amount, let it stand for 20 minutes for adsorption, and filter through titanium rod and filter element;
[0061] 3. After filtering, feed intake of famotidine according to 102% of the formula amount, stir and dissolve;
[0062] ④. Adjust the pH value to 5.2-5.4 with 10% aspartic acid solution treated with medicinal charcoal, add water for injection to the full amount, and stir evenly;
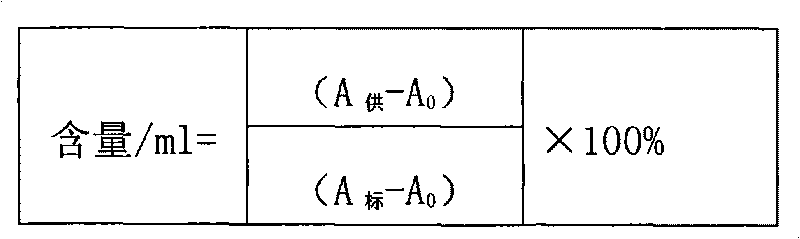
[0063] ⑤. Filter through 0.45 μm and 0.22 μm filter elements succe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com