Pharmaceutical composition for external use adopting glucocorticoid as active component
A technology for glucocorticoids and active ingredients, which is applied in the field of external pharmaceutical compositions, and can solve the problems of decreased content, leakage of composite hoses, convulsions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
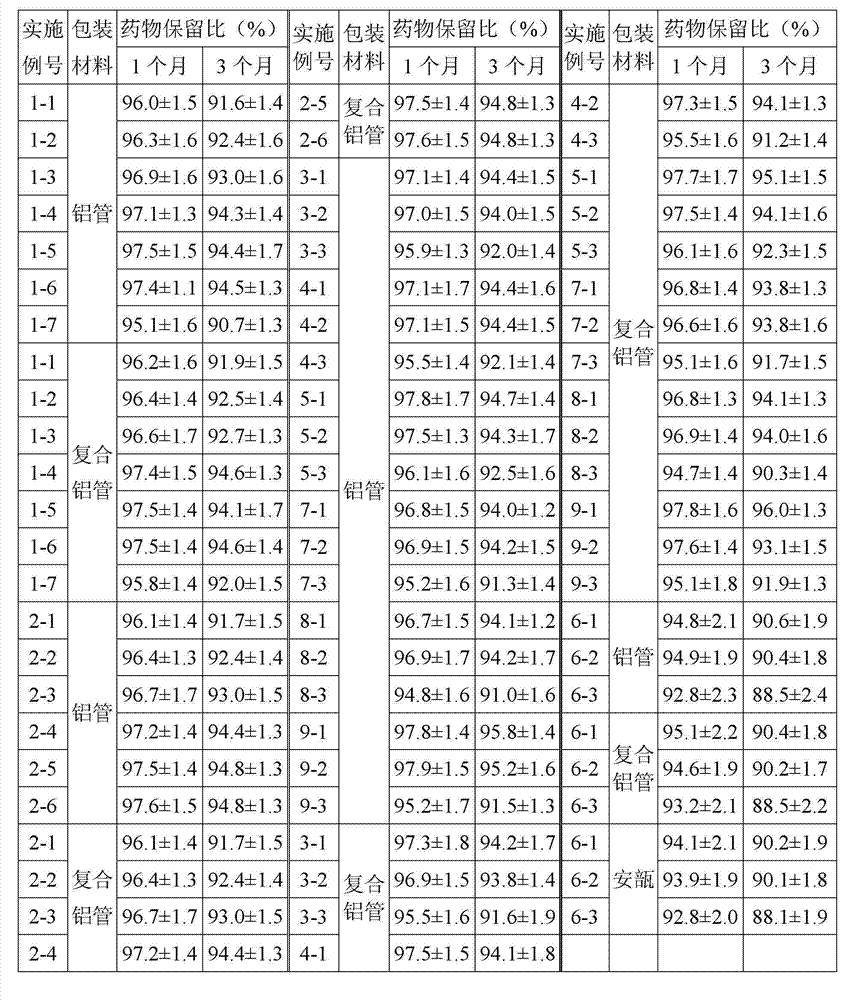
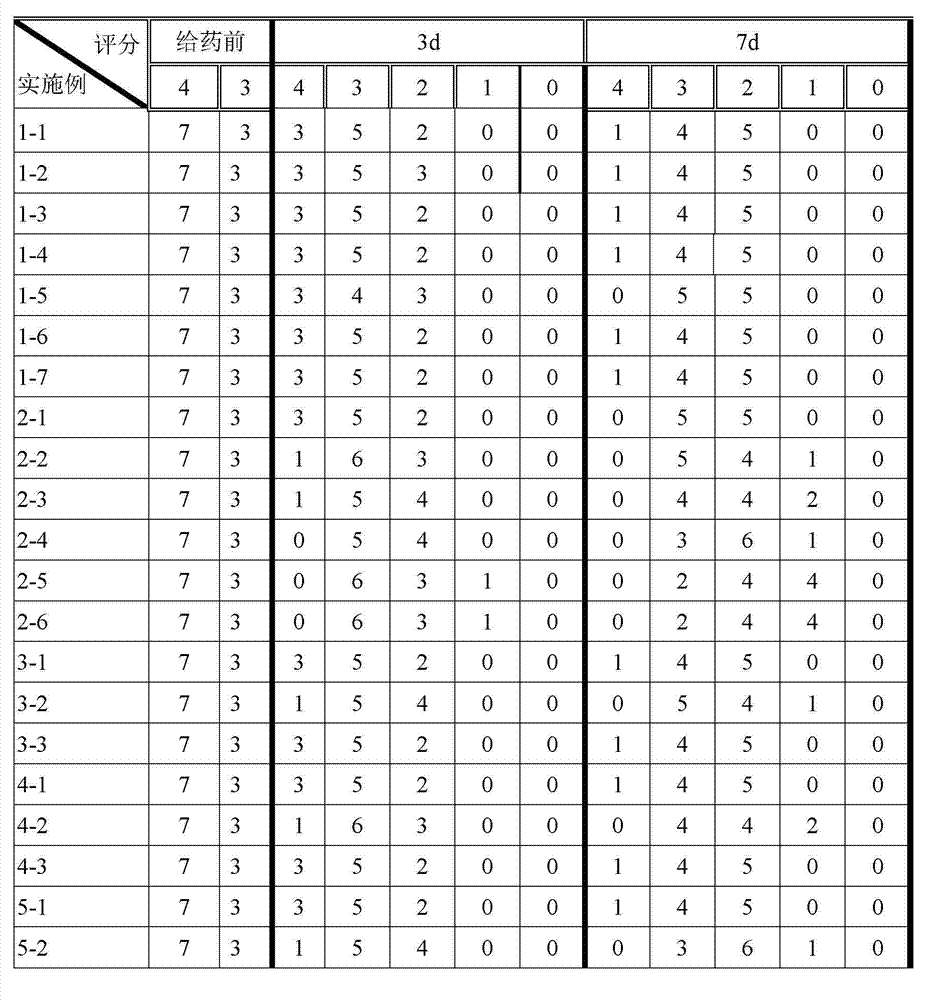
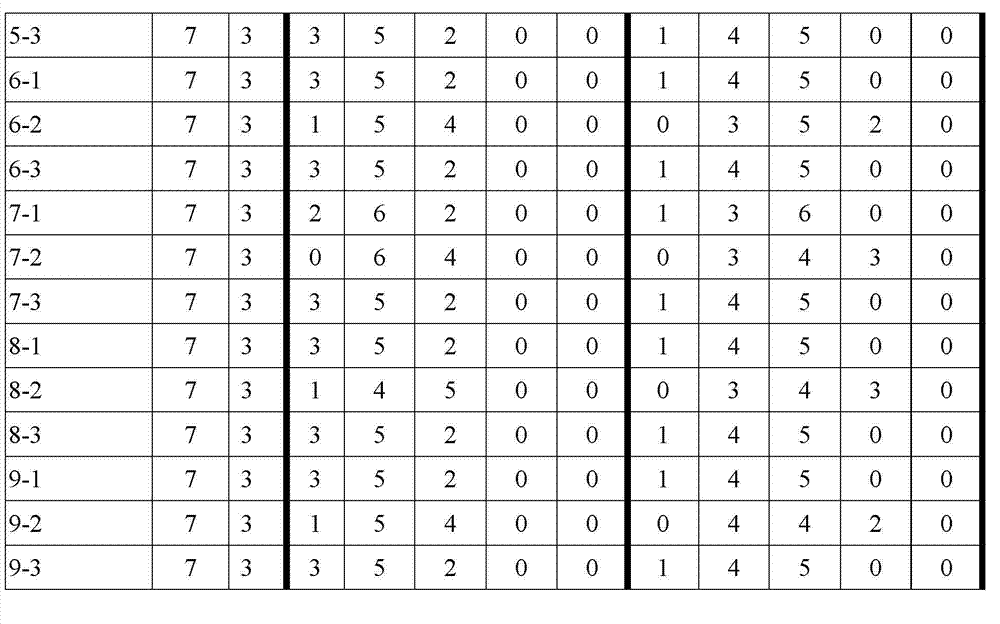
Image
Examples
Embodiment 1-1
[0045] Dexamethasone acetate 1g, white petrolatum 100g, stearyl alcohol 30g, liquid paraffin 30g,
[0046] Pingpingjia A-20 50g, glycerin 50g, propylene glycol 20g, ethyl p-hydroxybenzoate 1g,
[0047] Add edetate disodium 0.1g to 1000g with water for injection,
[0048] Accurately weighed according to the above ratio, the cream preparation process is as follows:
[0049] (1) Oil phase preparation: Take white petrolatum, stearyl alcohol, liquid paraffin, and Pingpingjia A-20 in a container, heat until melting, and keep the temperature at 90°C;
[0050] (2) Water phase preparation: Dissolve edetate disodium in water for injection, disperse the main drug evenly in glycerin and propylene glycol, add the aqueous solution of edetate disodium, ethyl p-hydroxybenzoate, heat, and stir well The temperature is kept at 90°C;
[0051] (3) Convergence: slowly add the oil phase prepared in step (1) into the water phase prepared in step (2), stir, keep the temperature at 80°C, stir for 30...
Embodiment 1-2
[0053] According to the recipe and process of Example 1-1, the amount of disodium edetate was changed to 0.25g.
Embodiment 1-3
[0055] According to the recipe and process of Example 1-1, the amount of edetate disodium was changed to 1 g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com