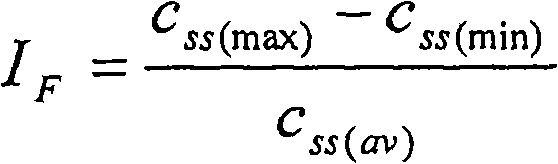Neramexane MR matrix tablet
A technology of neramexane, release agent, applied in the direction of anti-inflammatory agent, pill delivery, antifungal agent, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0091] The present invention is further illustrated by the following examples, which, however, should not be construed as limiting the scope of the invention.
Embodiment 1
[0093] Example 1: Preparation of neramexin modified-release matrix tablet
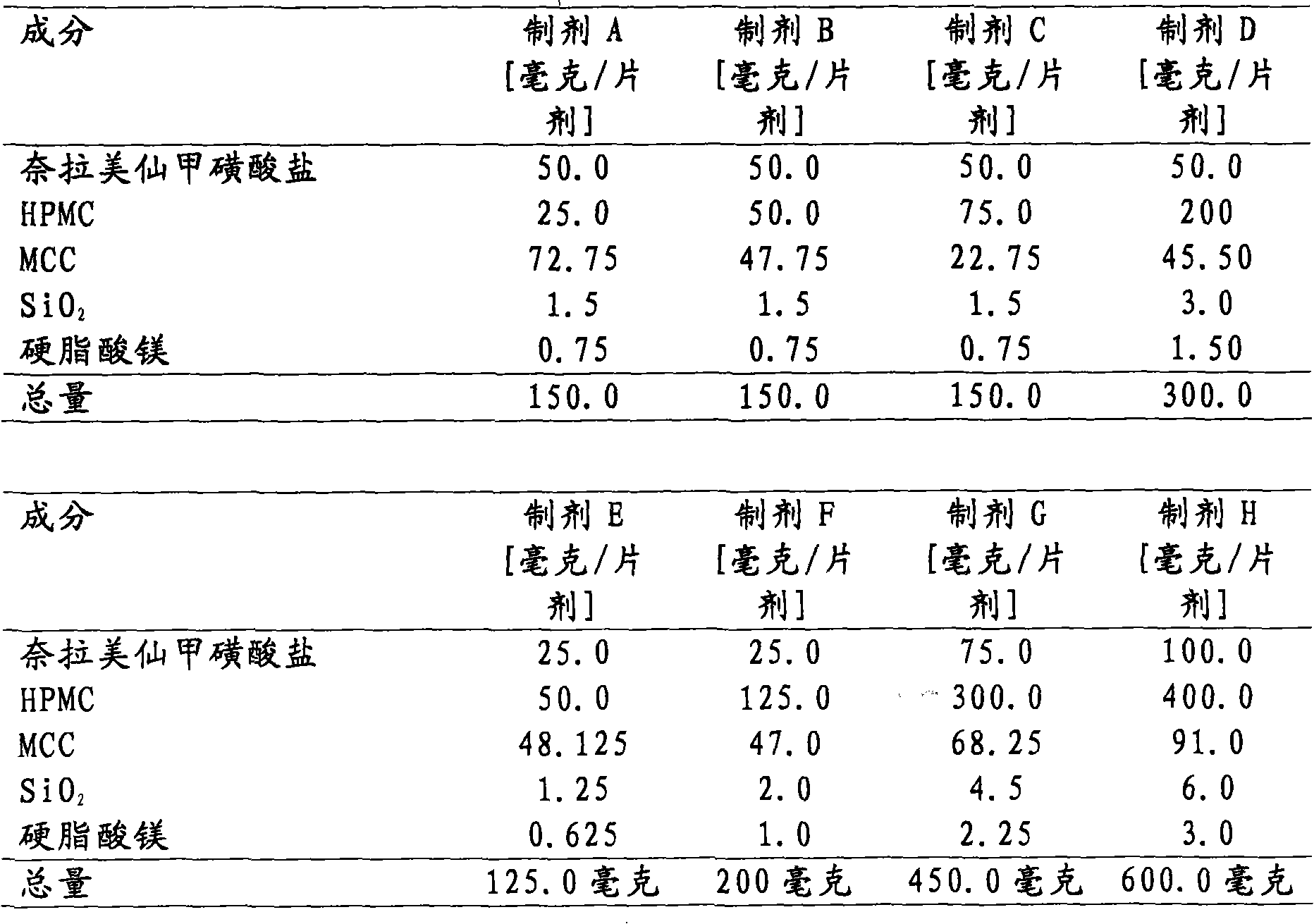
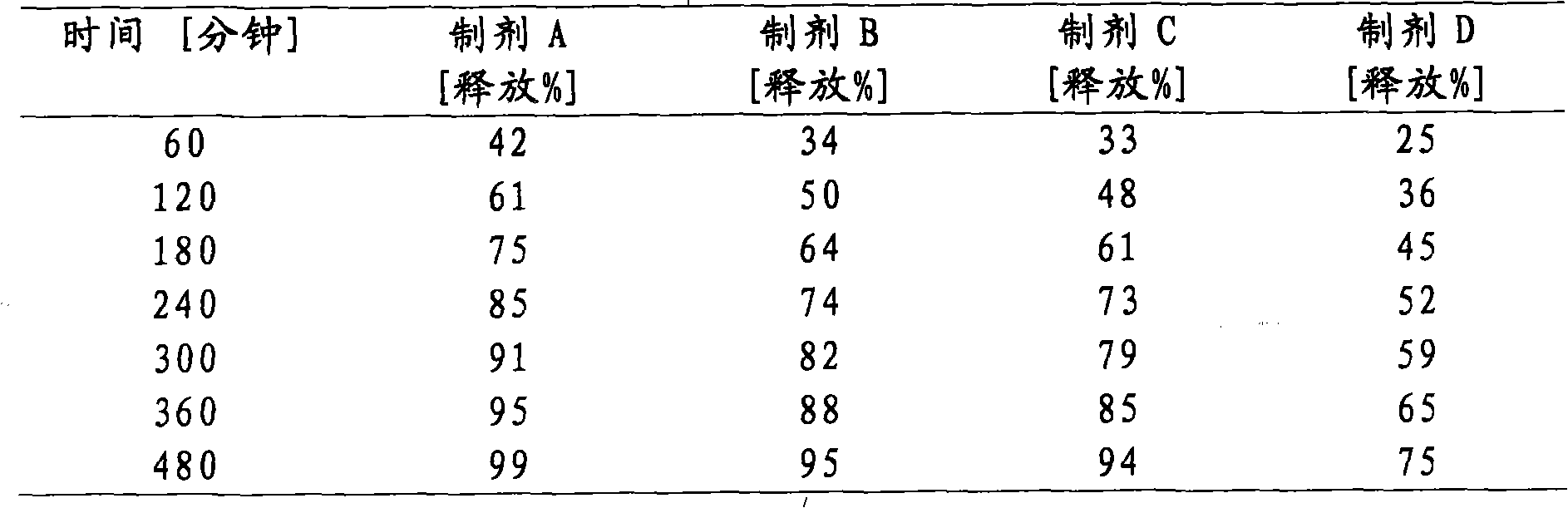
[0094] Matrix tablets containing about 25 mg or 50 mg or 75 mg or 100 mg of neramexin mesylate are prepared as follows. Appropriate amount of neramexin mesylate, hydroxypropyl methylcellulose (HPMC, this example is Methocel K 100M CR), microcrystalline cellulose (MCC, this example is Avicel PH 102), magnesium stearate and gum SiO2 (SiO 2 , in this case Aerosil 200) were weighed and blended using a free-fall blender (Bohle PTM 200). Or an appropriate amount of neramexin mesylate, hydroxypropyl methylcellulose, microcrystalline cellulose, magnesium stearate and colloidal silicon dioxide is sieved before blending using a free-fall blender. The appropriate amount for each batch is calculated based on the target content of each dosage unit shown in Table 1. The optical characteristics of the powder blend showed no lack of homogeneity such as flakes, agglomerates or tendency to separate. All blends showe...
Embodiment 2
[0098] Example 2: Coating of Neramexin Modified Release Matrix Tablets
[0099] The matrix tablet prepared in Example 1 was coated with a white water-soluble coating composition Sepifilm LP 770 white using a porous or non-porous standard pan coater under controlled air. Tablets were weighed and dedusted prior to coating. The coating dispersion was then sprayed onto the tablets using a 1.0 mm nozzle. The temperature of the tablet cores during coating was between 34 and 39°C. The inlet temperature was between 59 and 64°C and the spray rate was about 40 to 53 grams / minute. Spraying was continued until the tablet weight gain was about 4%. The optical appearance of the coated tablets was excellent. Visibly free from sticking, the surface is smooth, bright and extremely uniform without any cracks or damage.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com