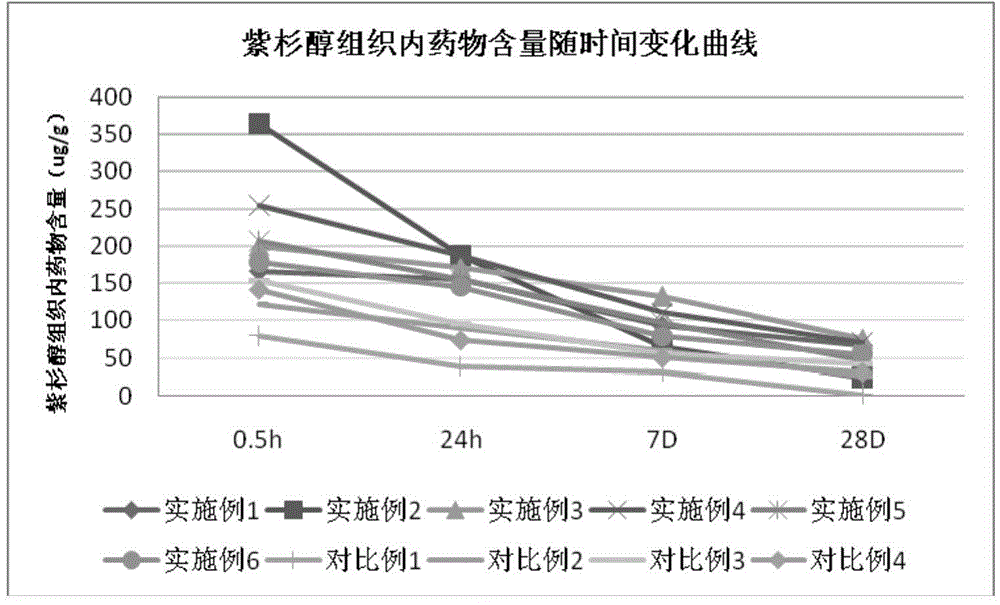
Patents
Literature
62 results about "Drug coated balloon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
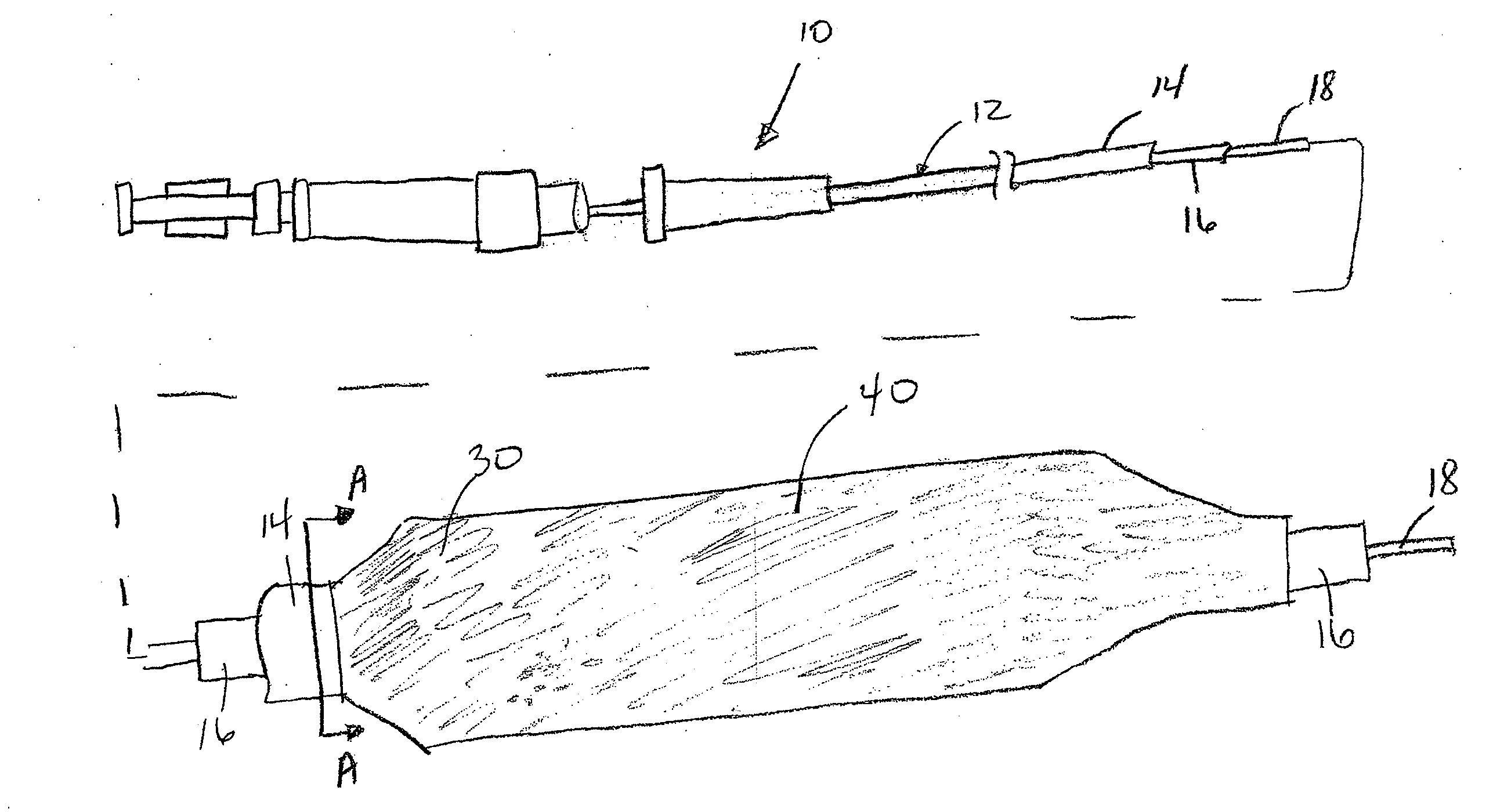
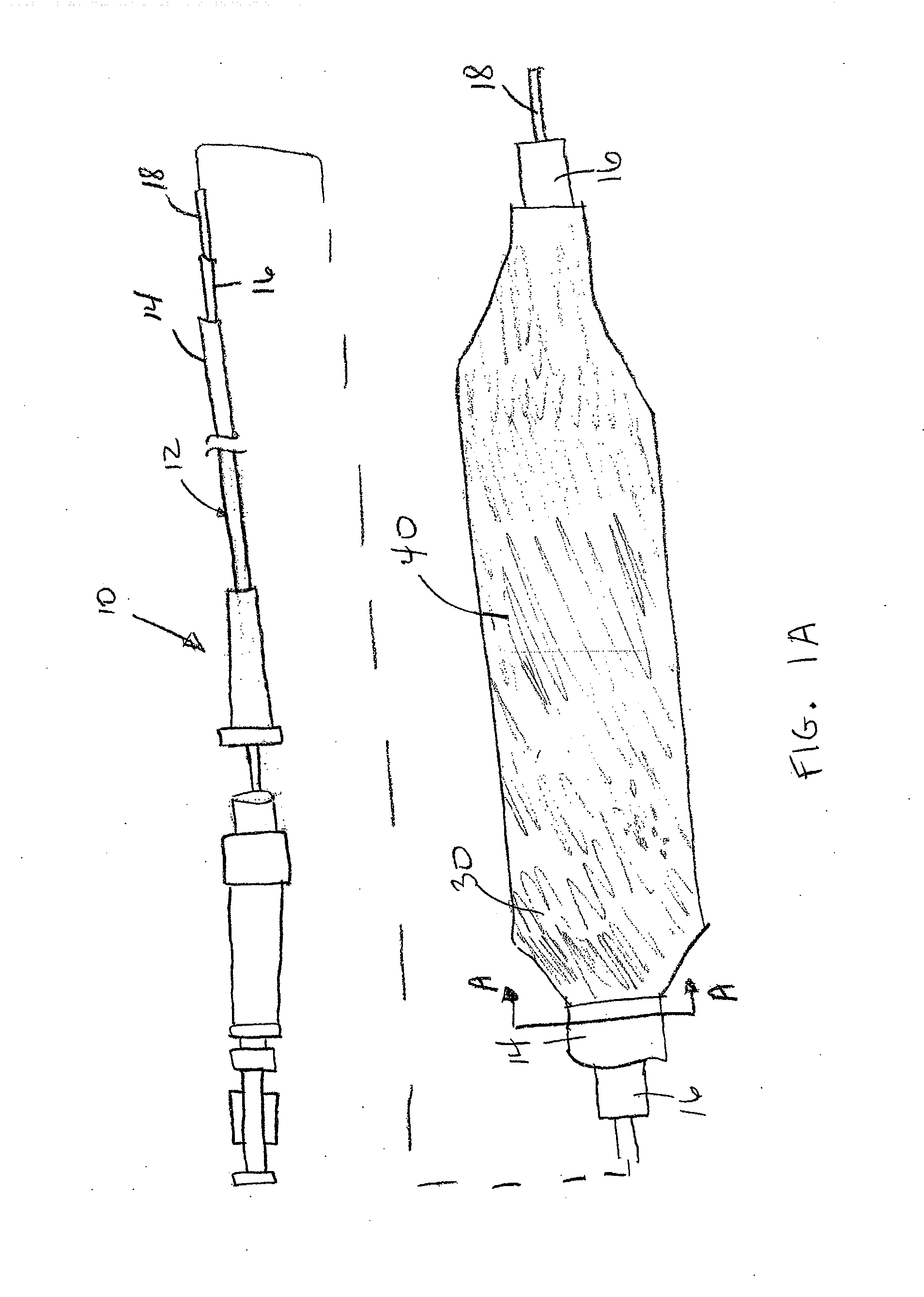
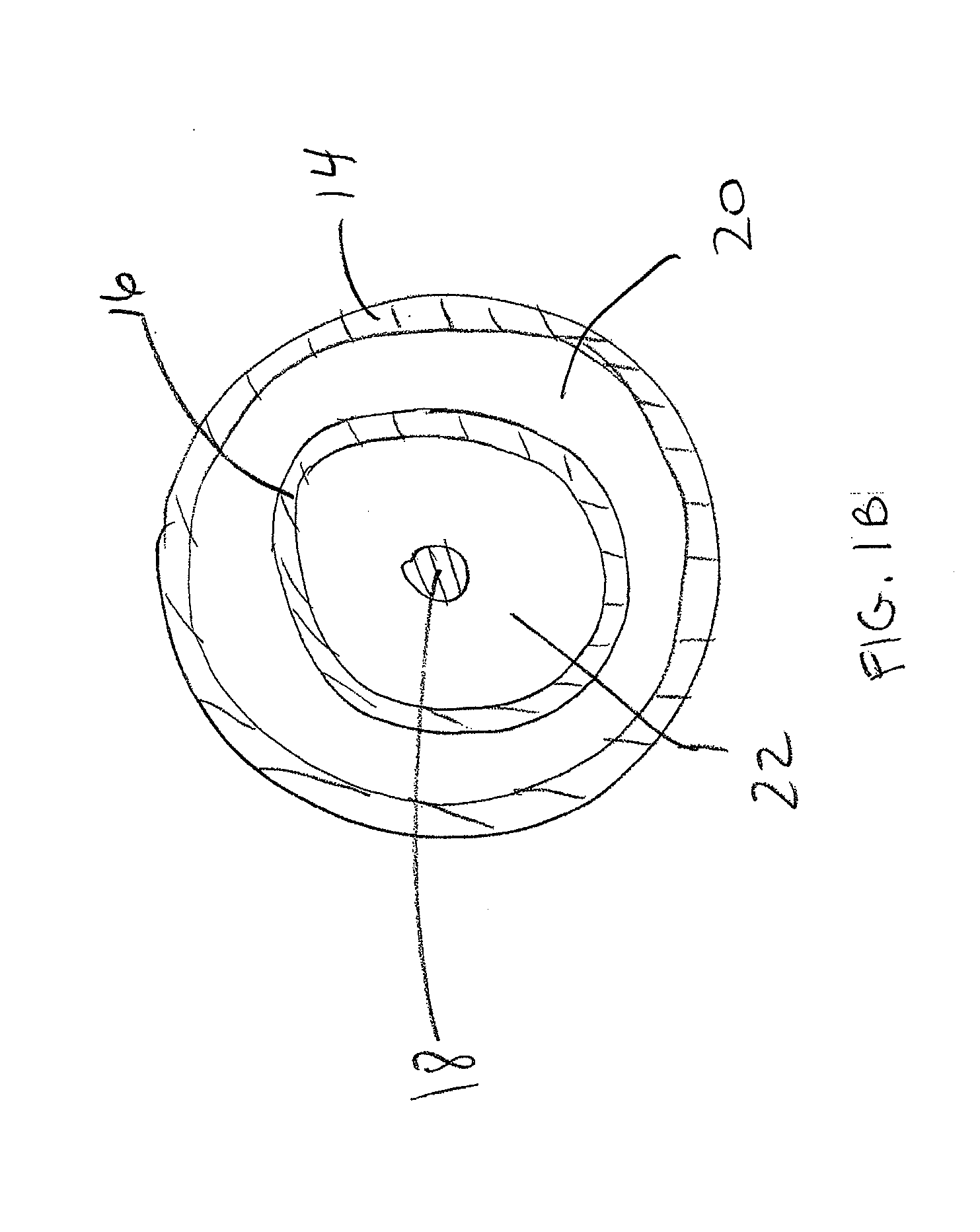
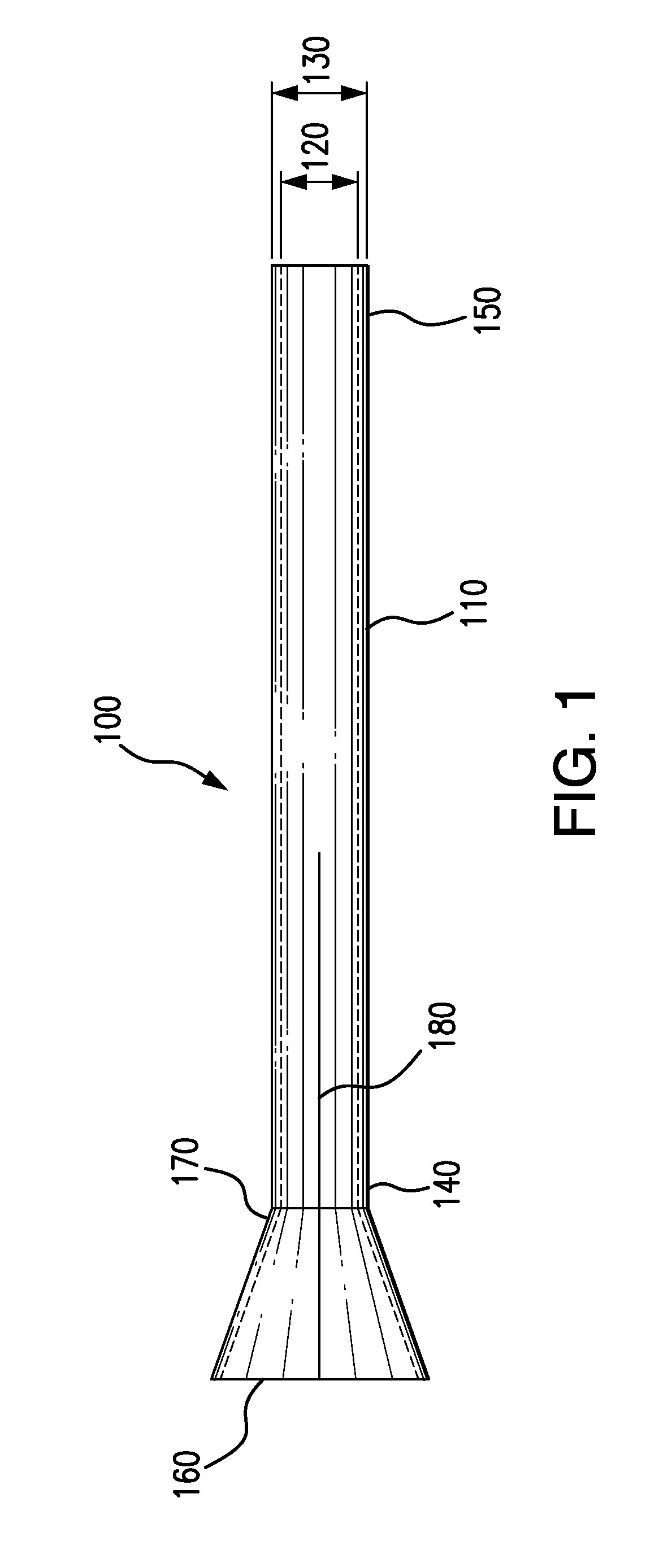
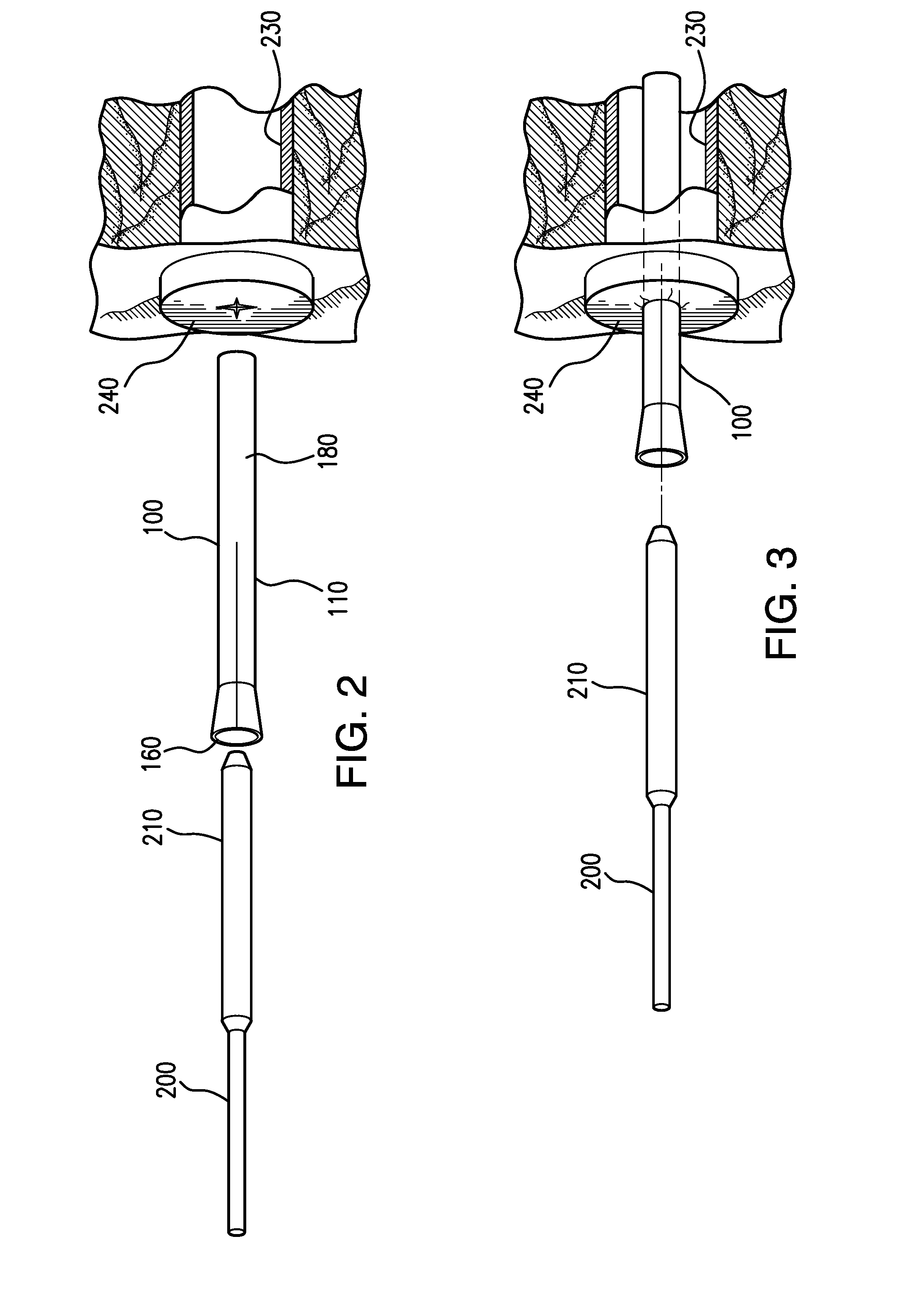
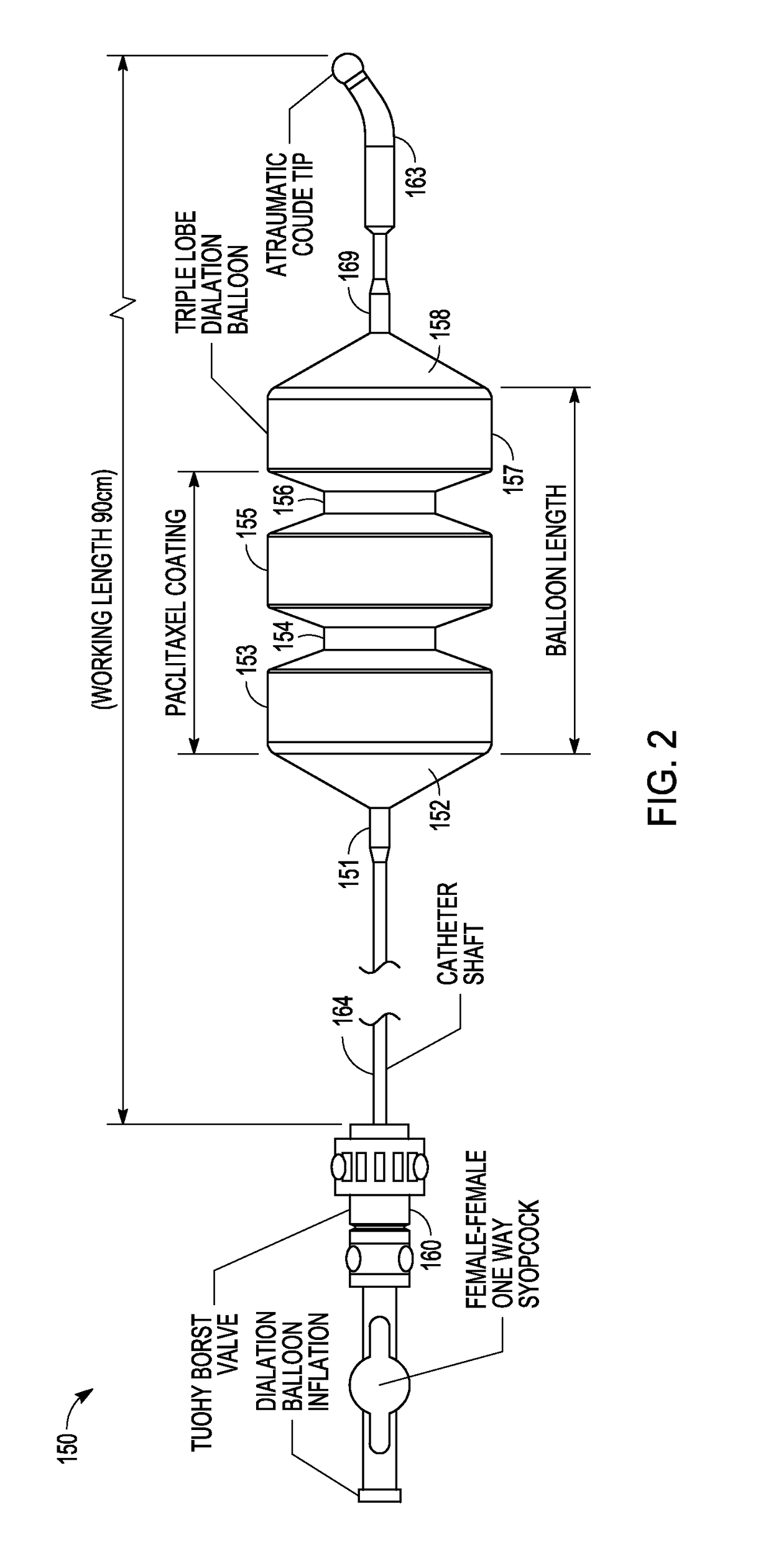
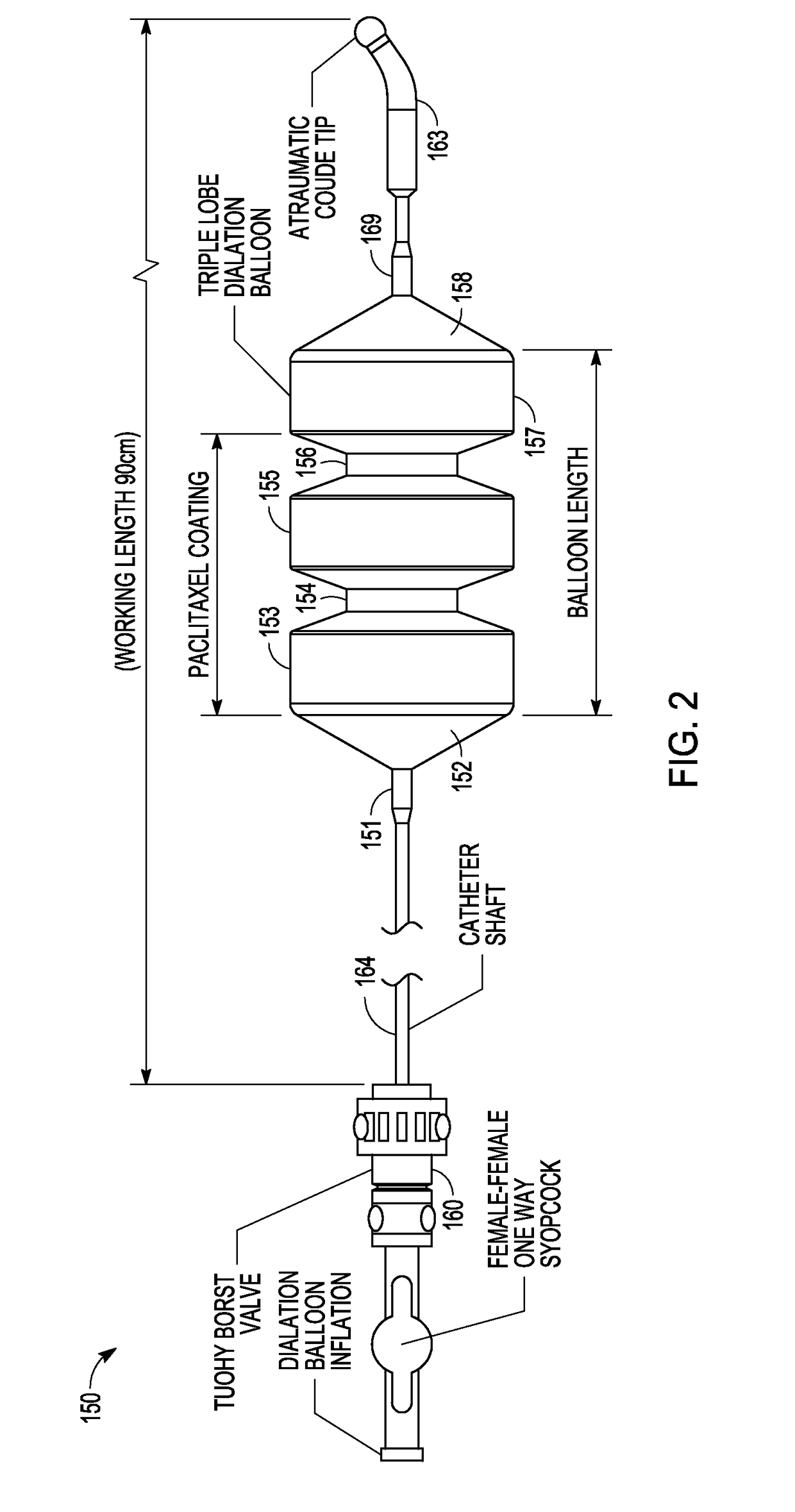
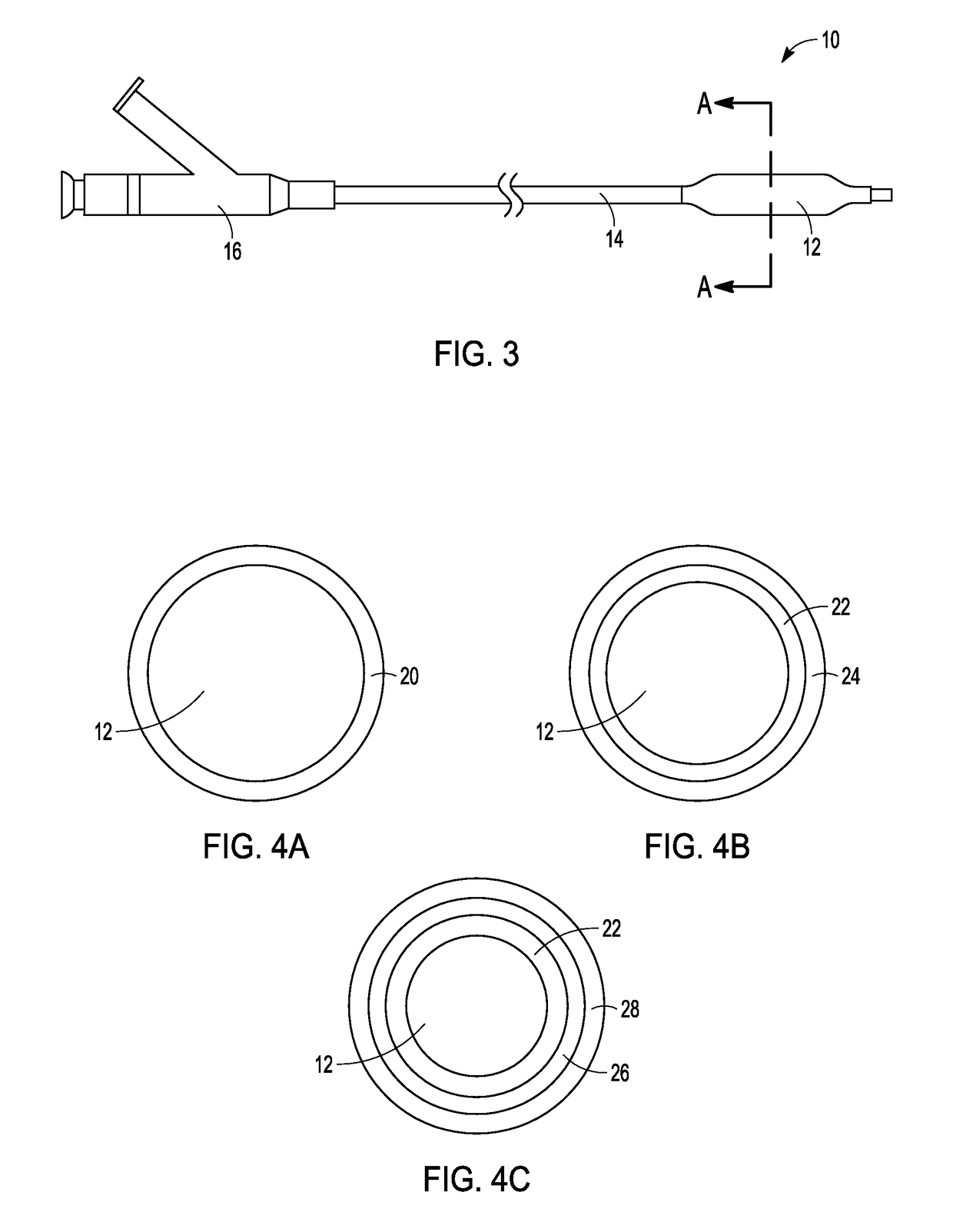
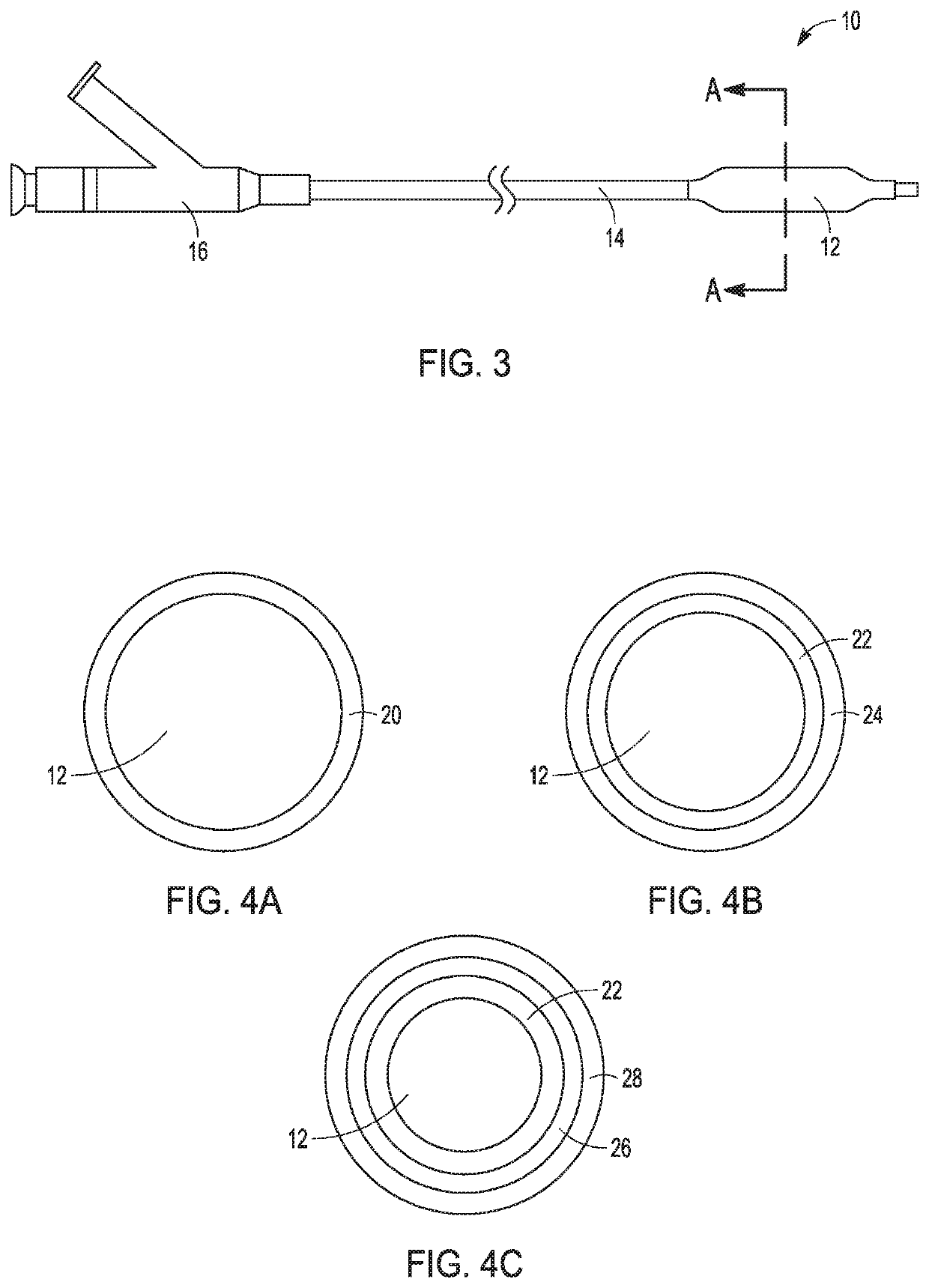
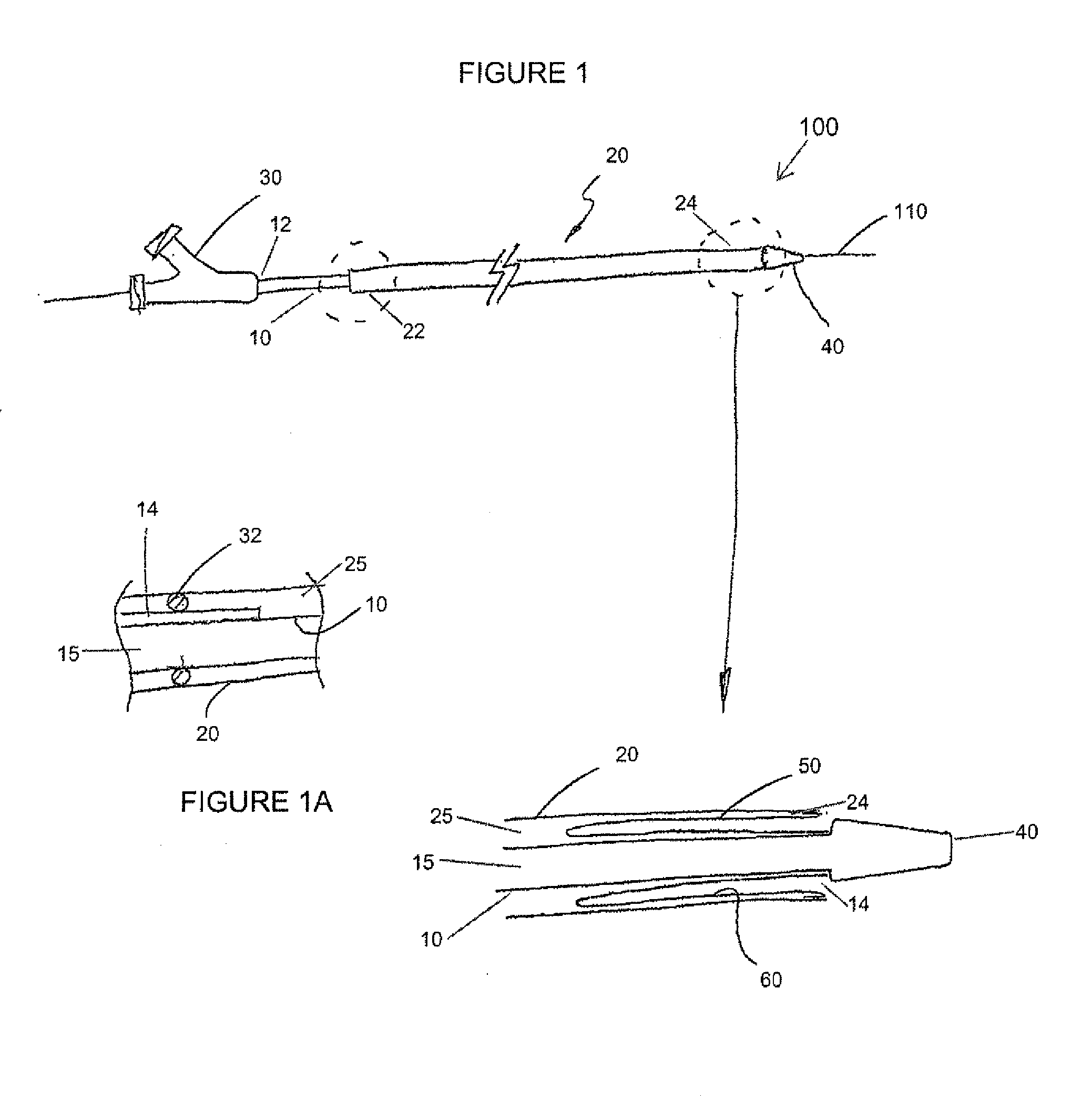
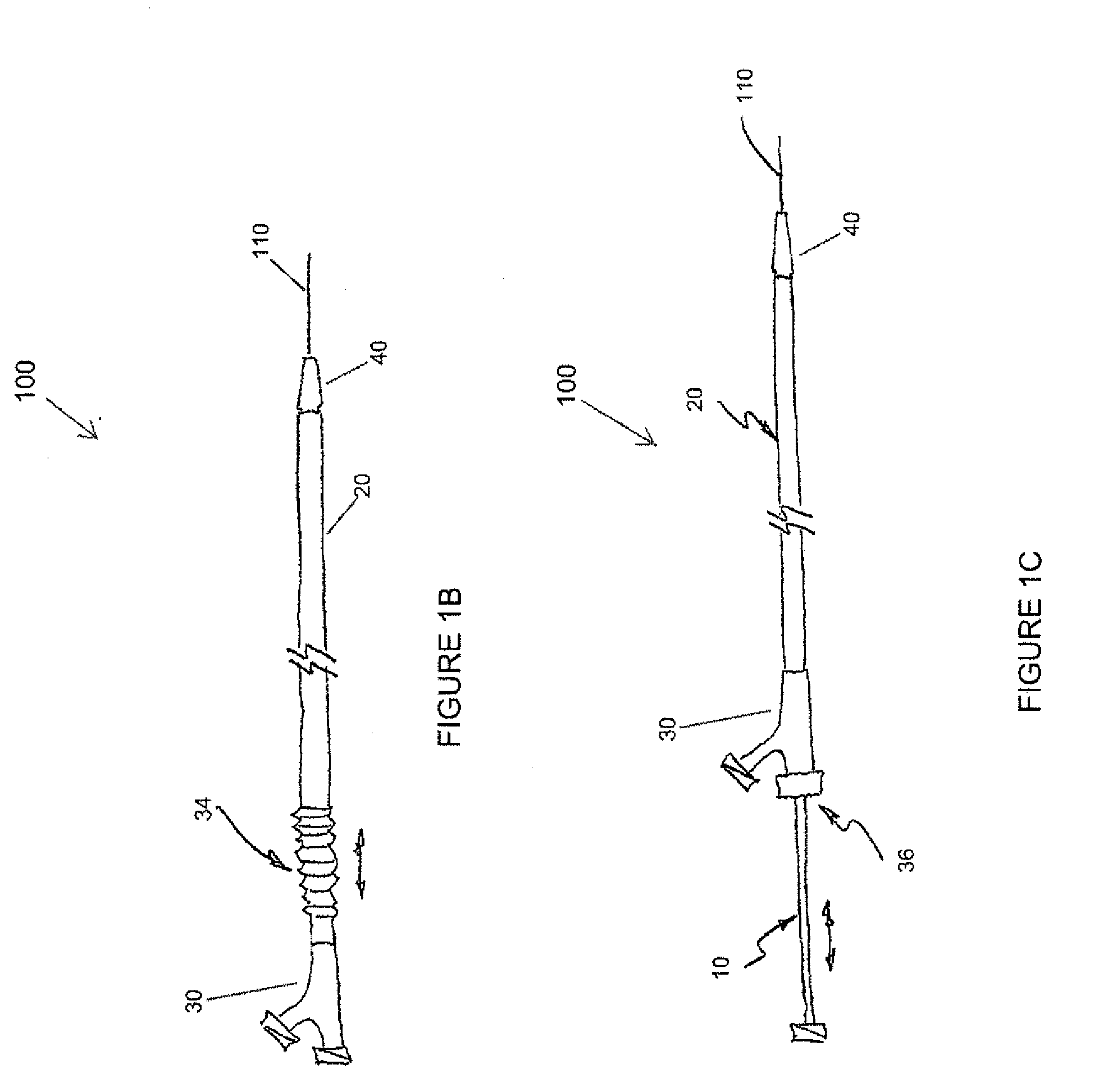
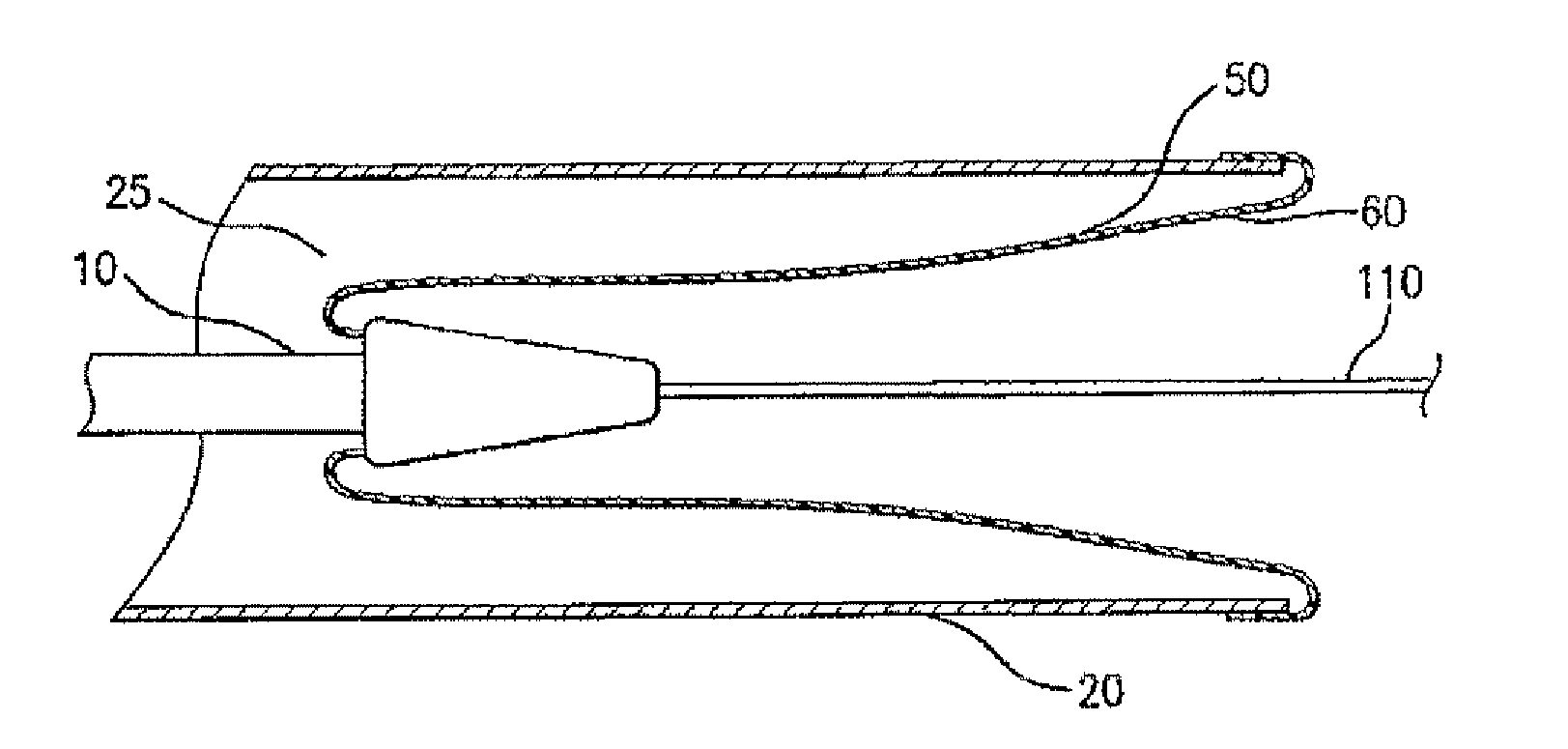
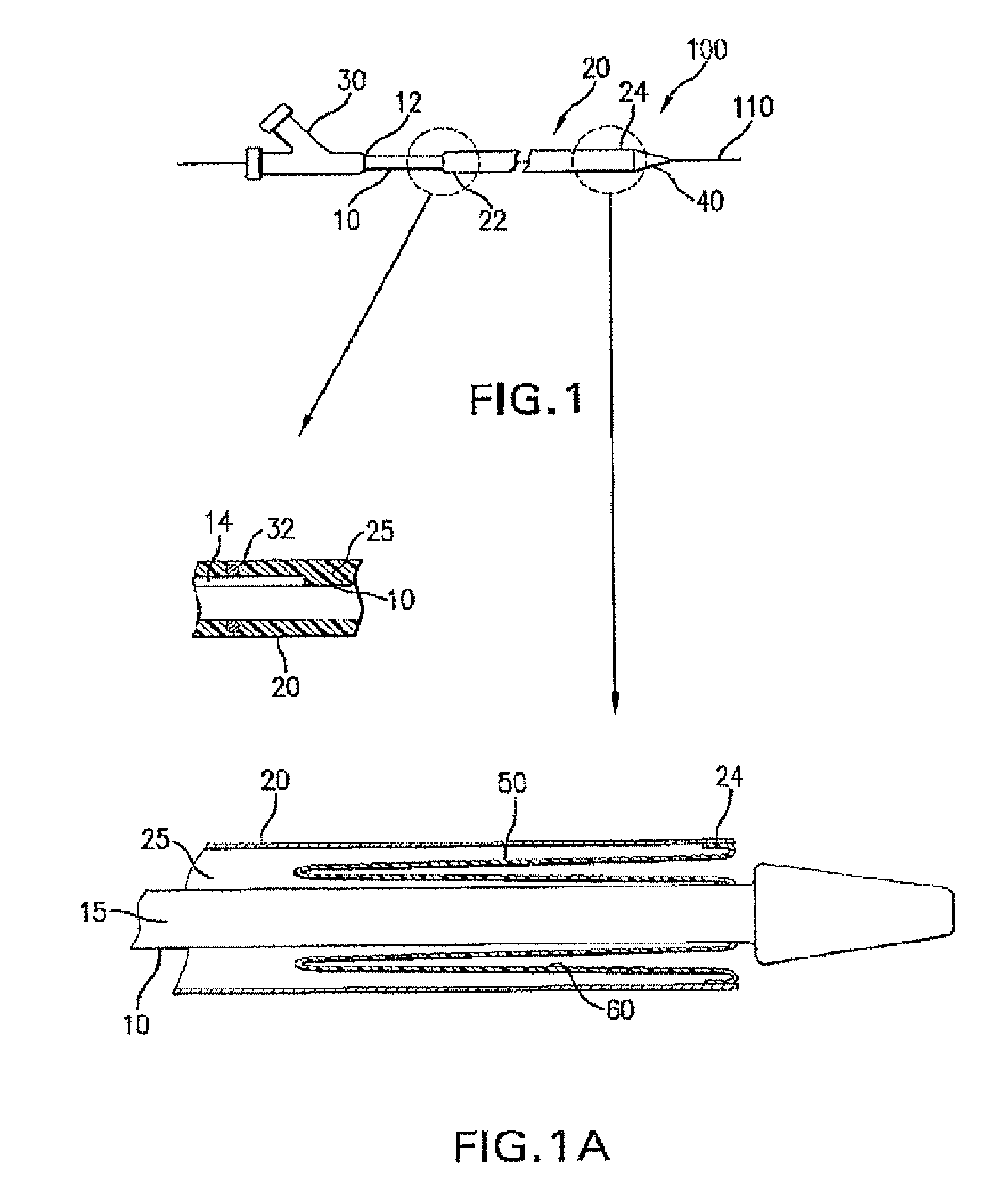
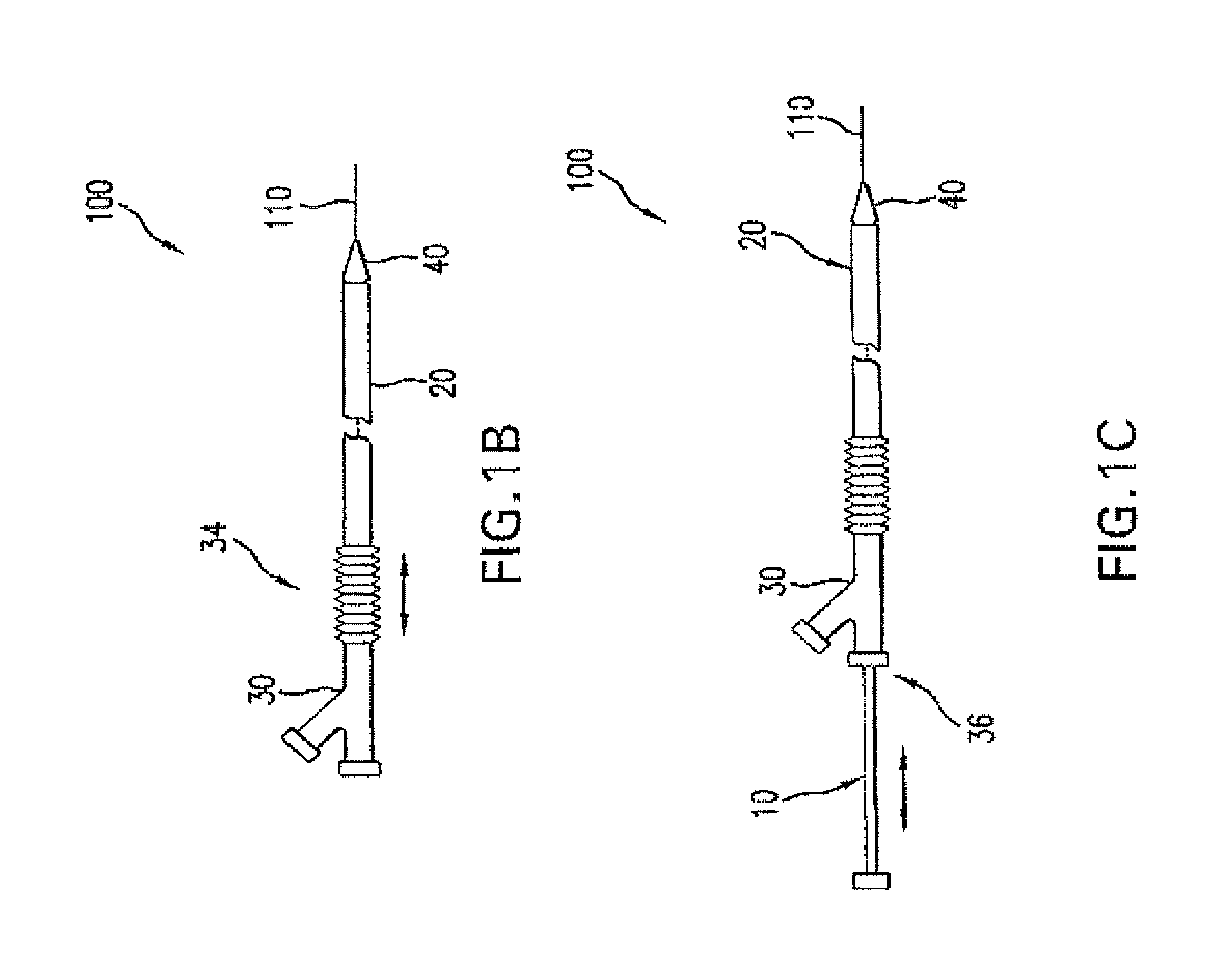
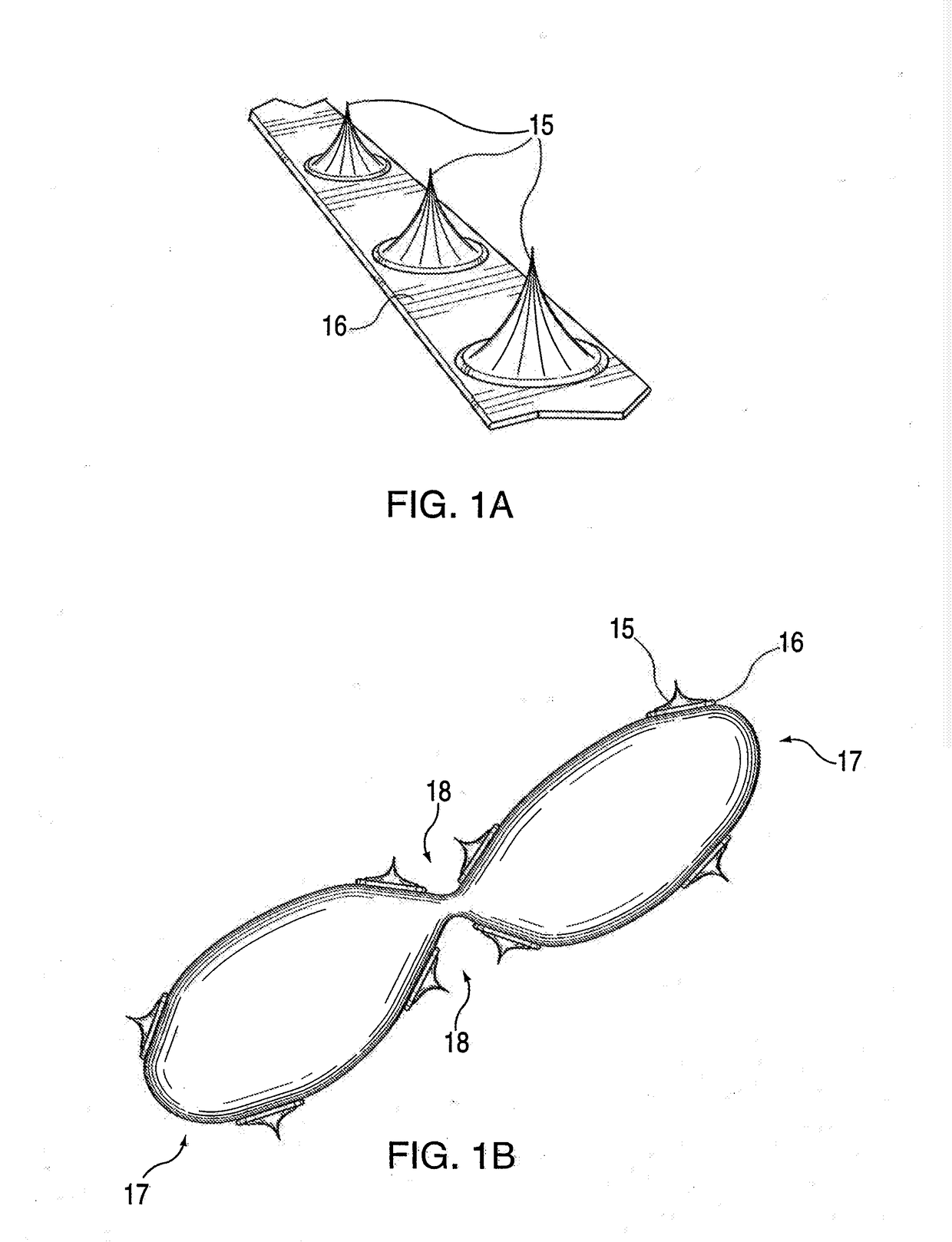
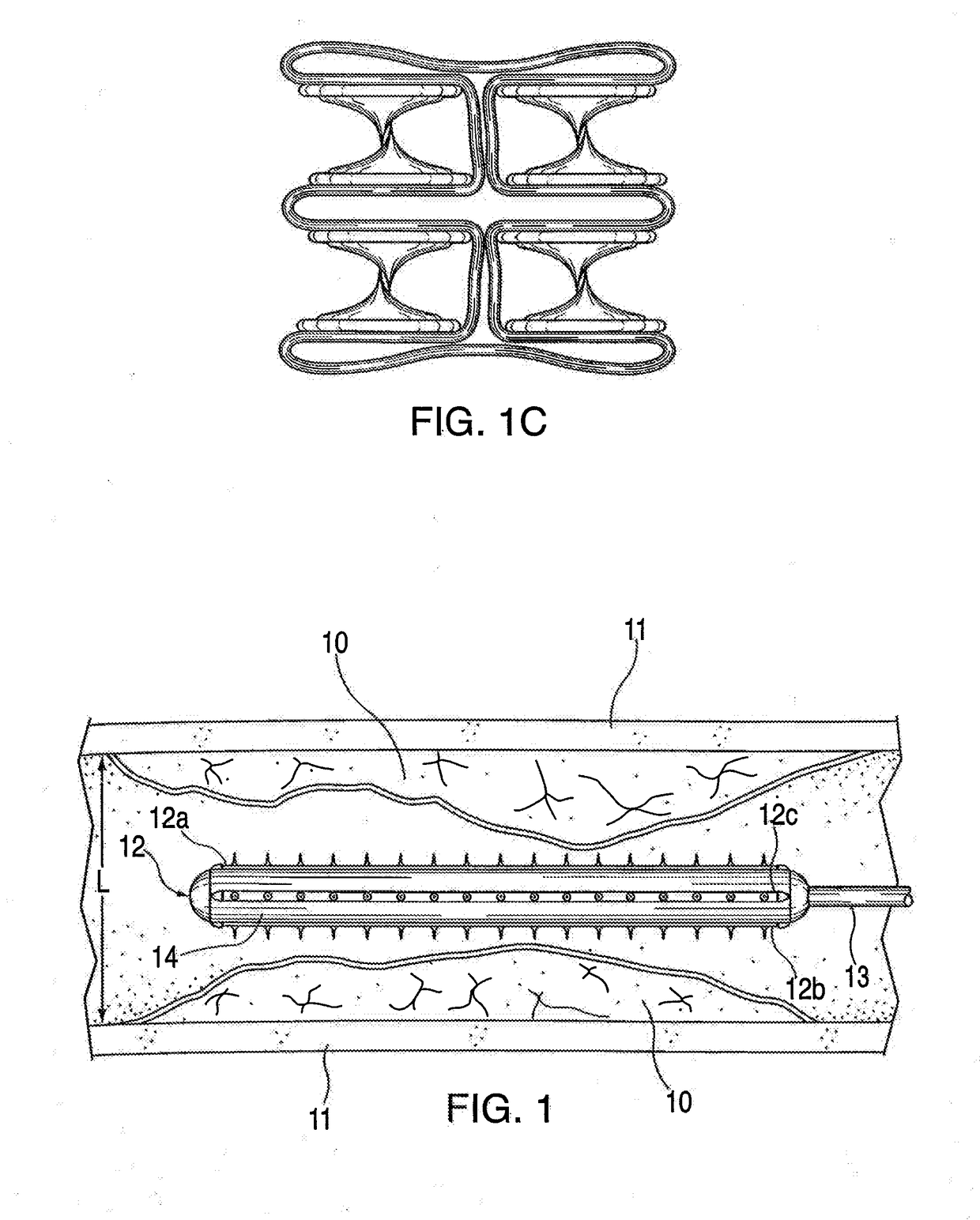
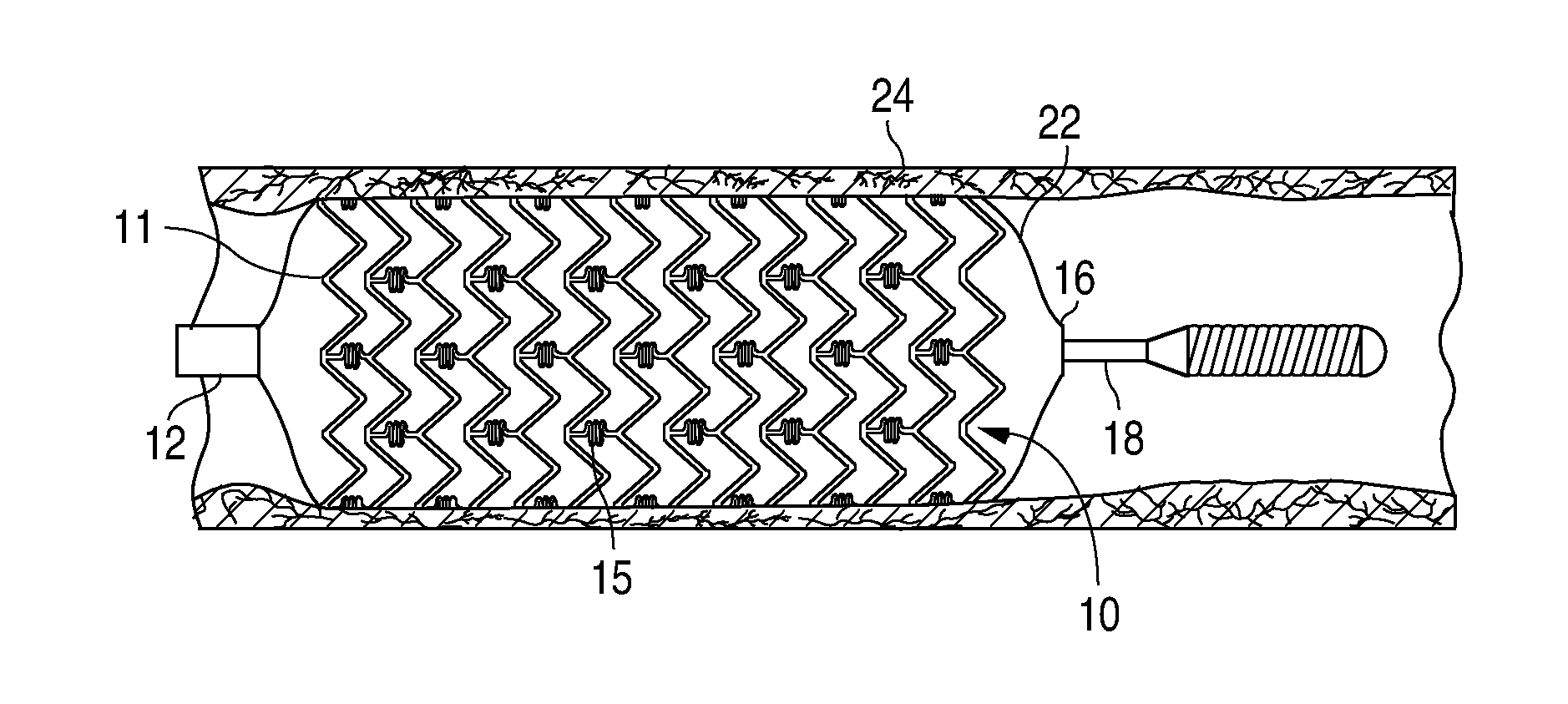
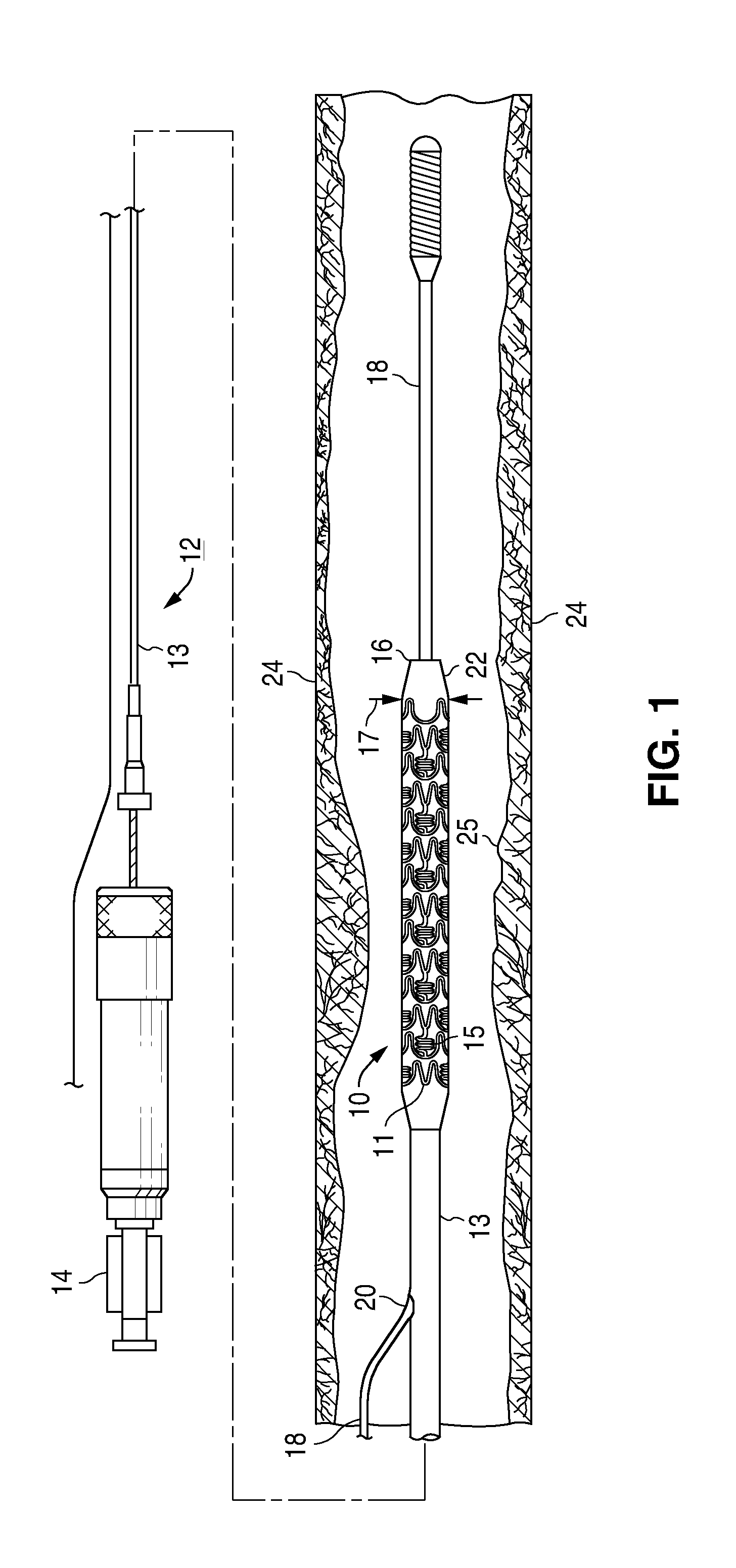
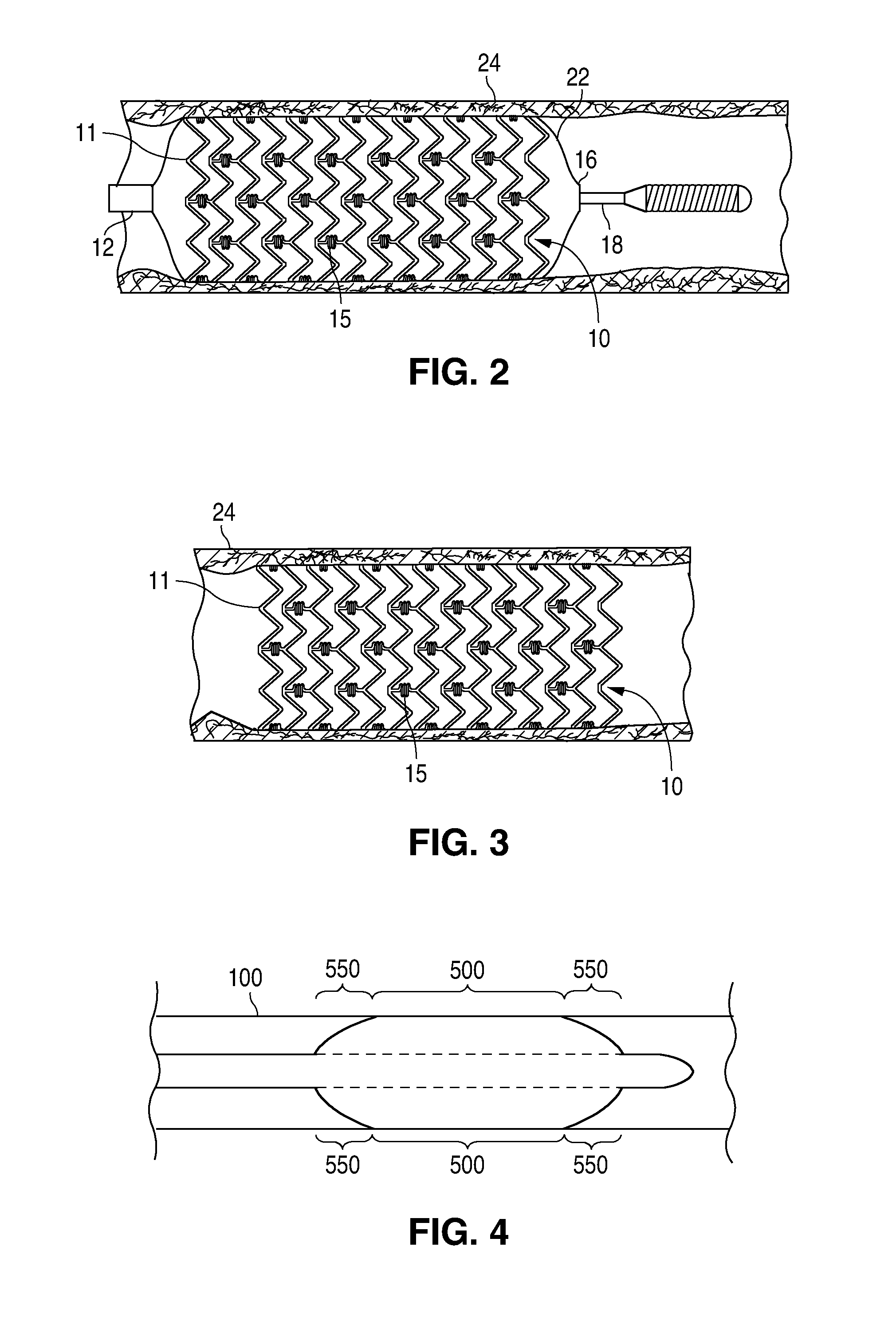
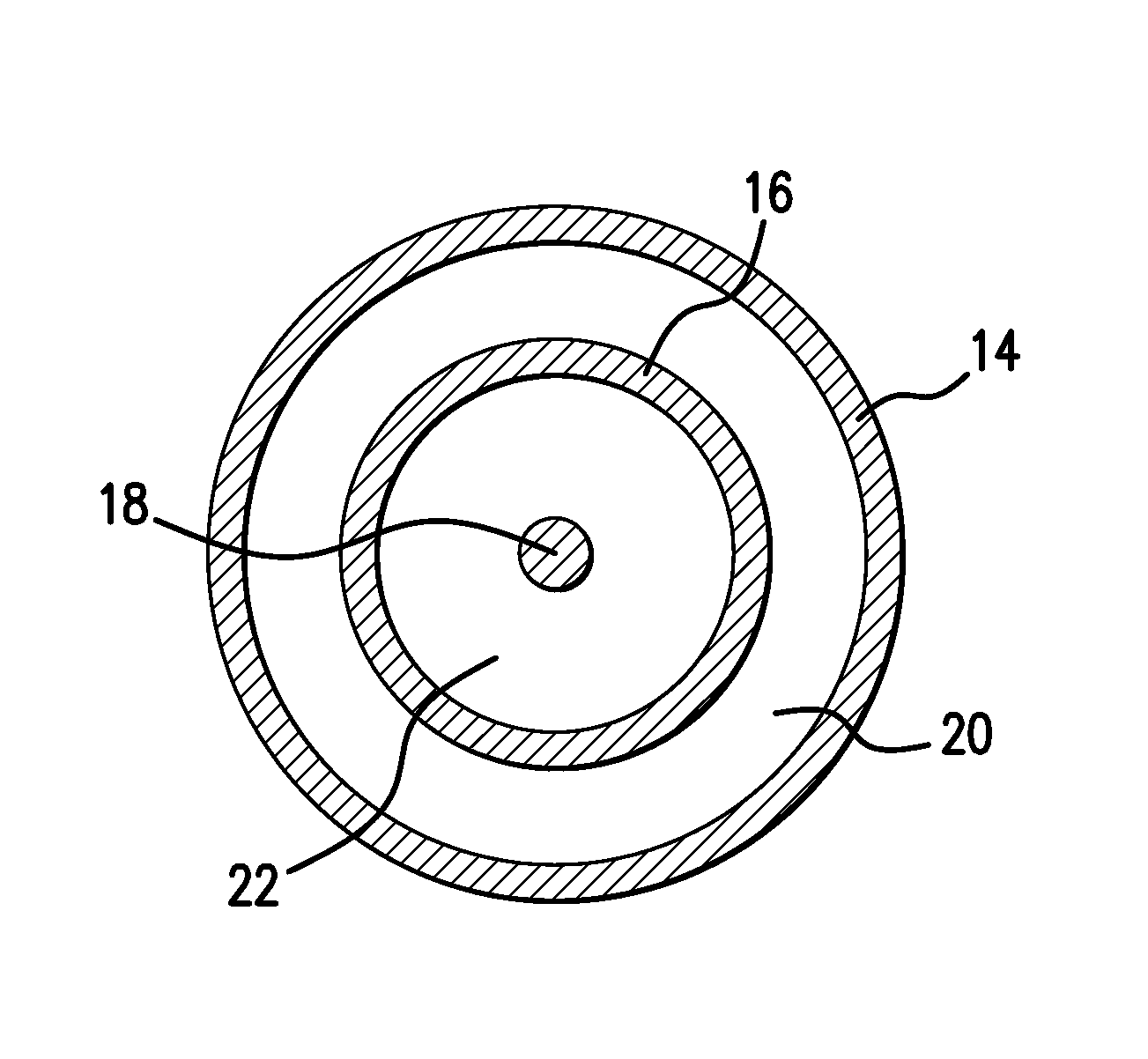
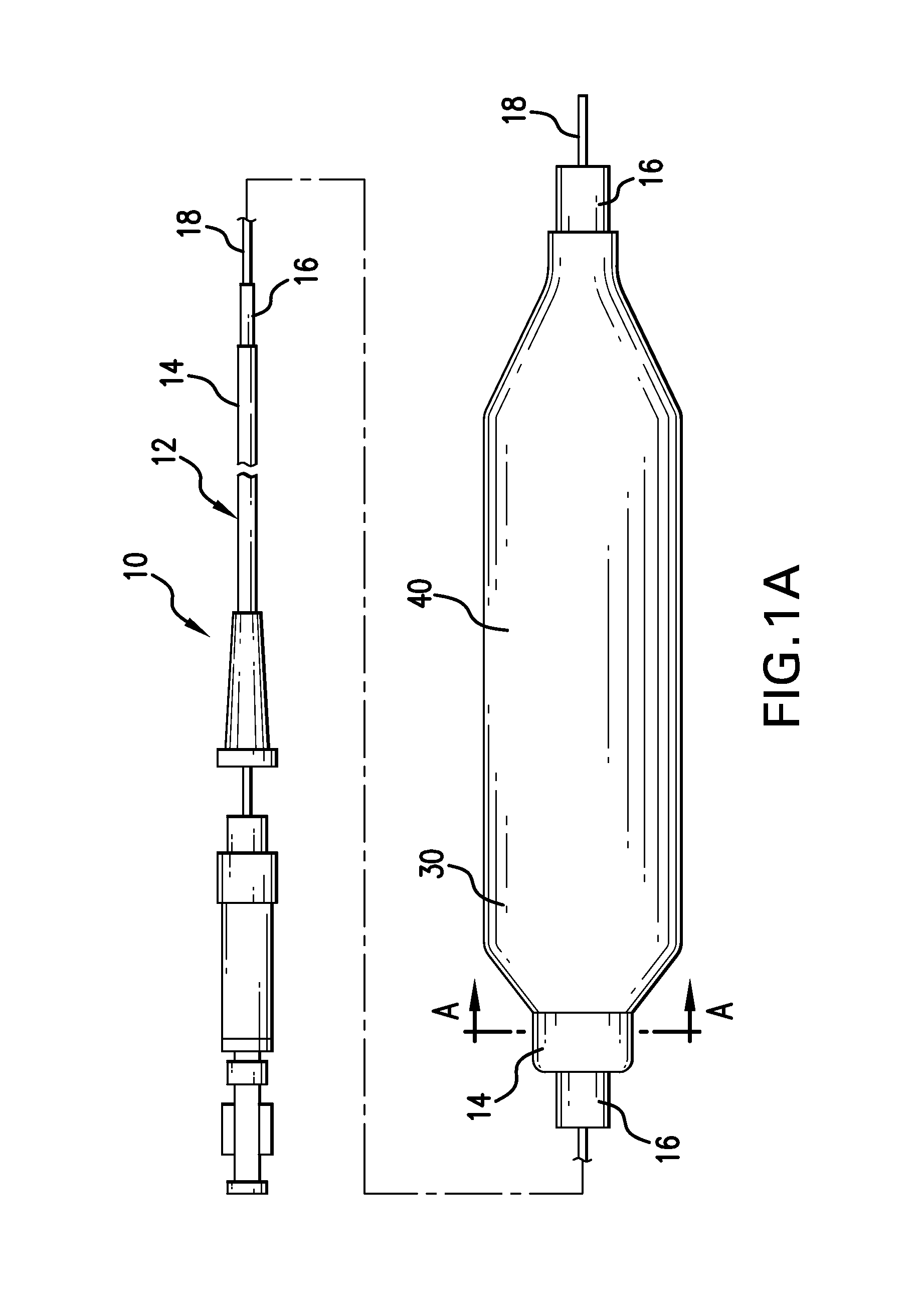
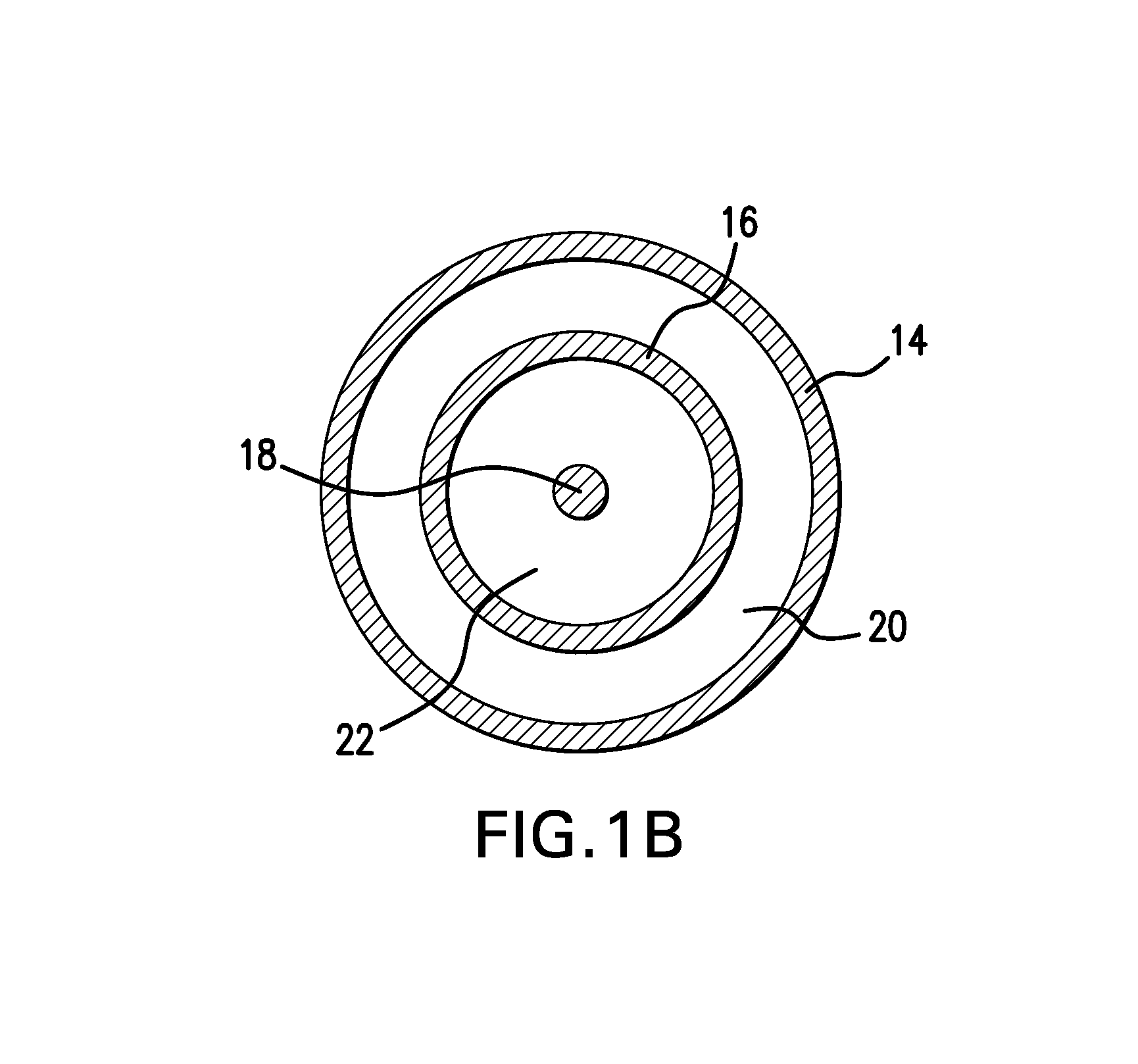
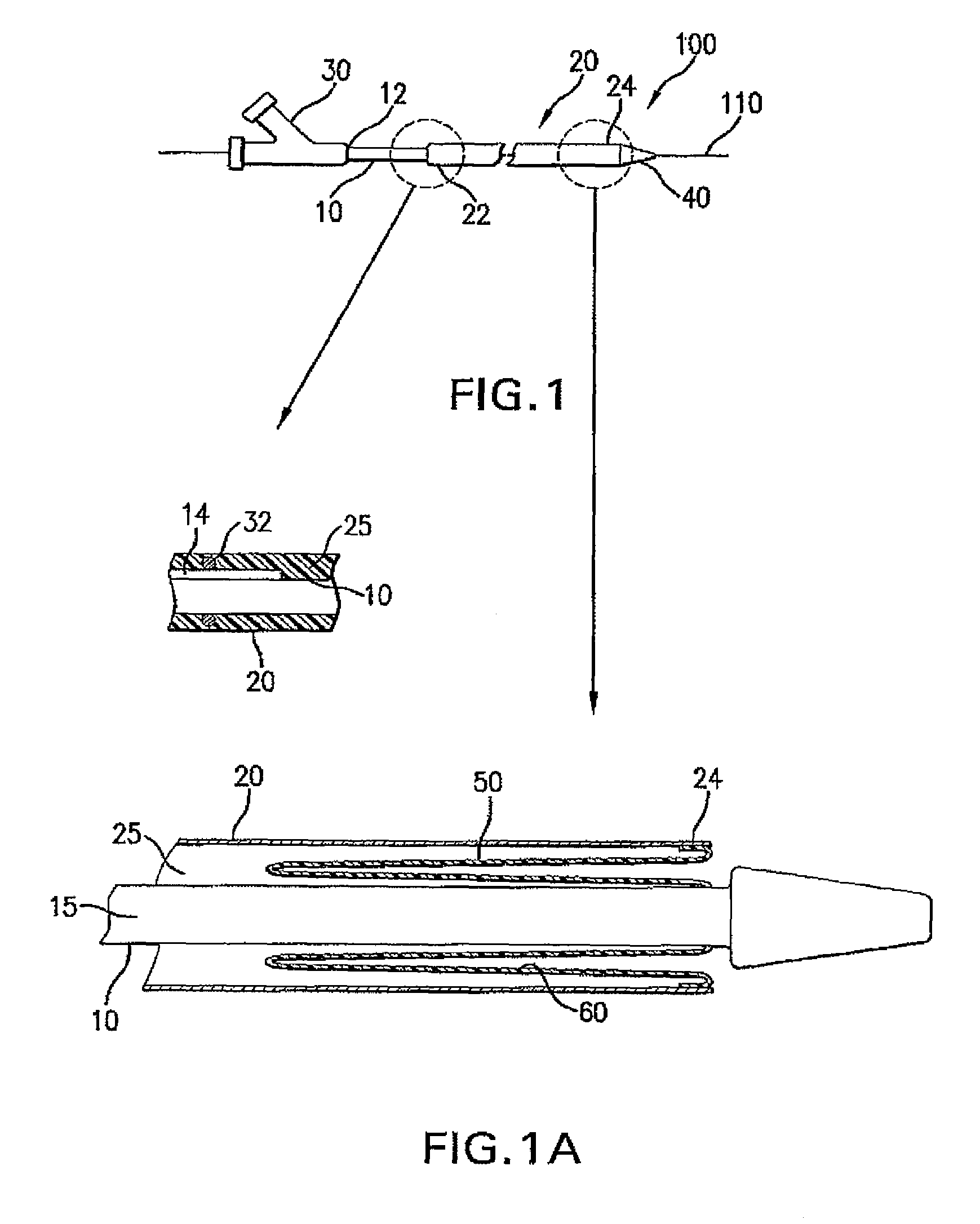
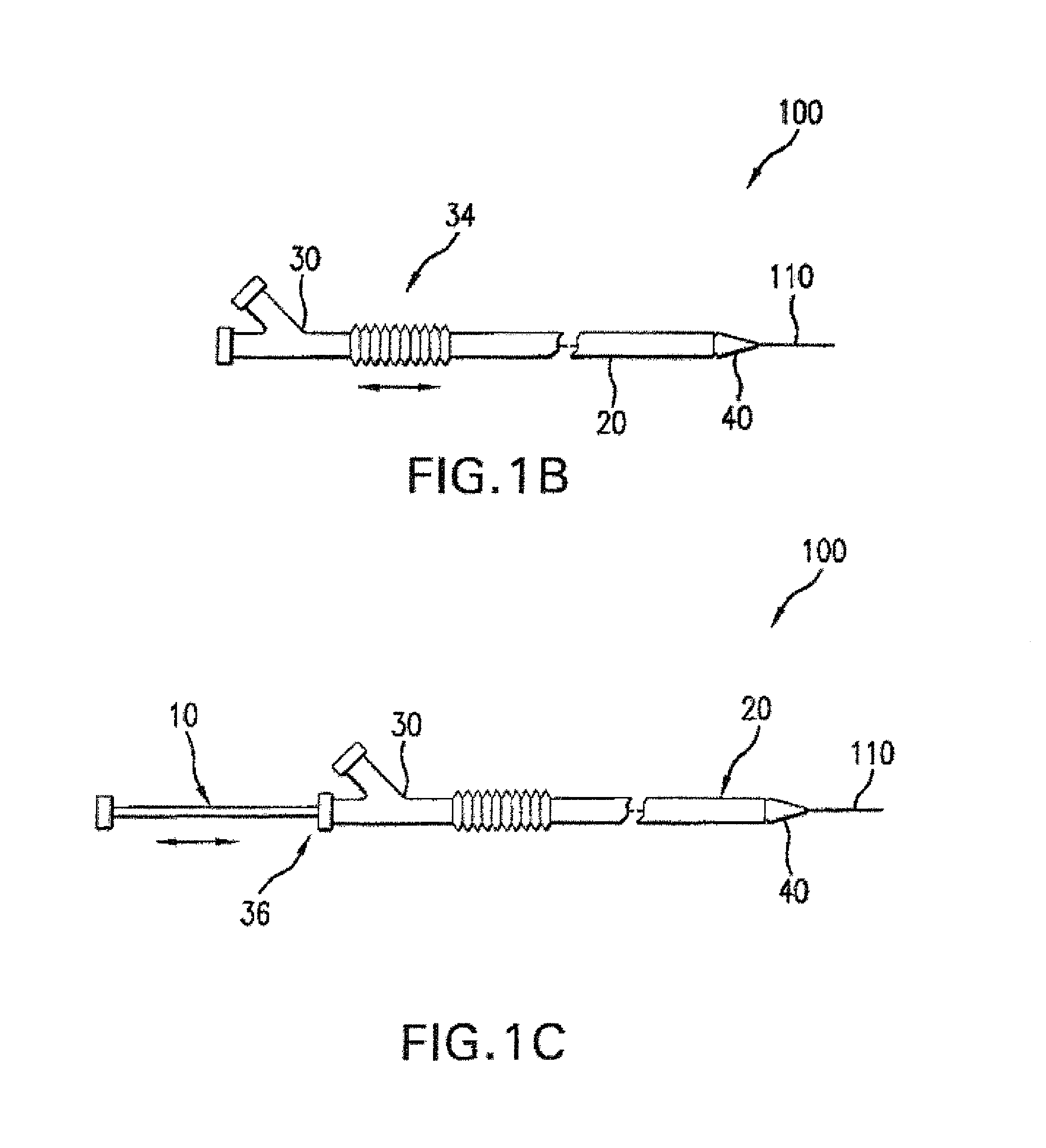
Adjustable-length drug delivery balloon
ActiveUS20090105686A1Inhibit and prevent restenosisDesirable lengthStentsBalloon catheterProsthesisBalloon dilatation
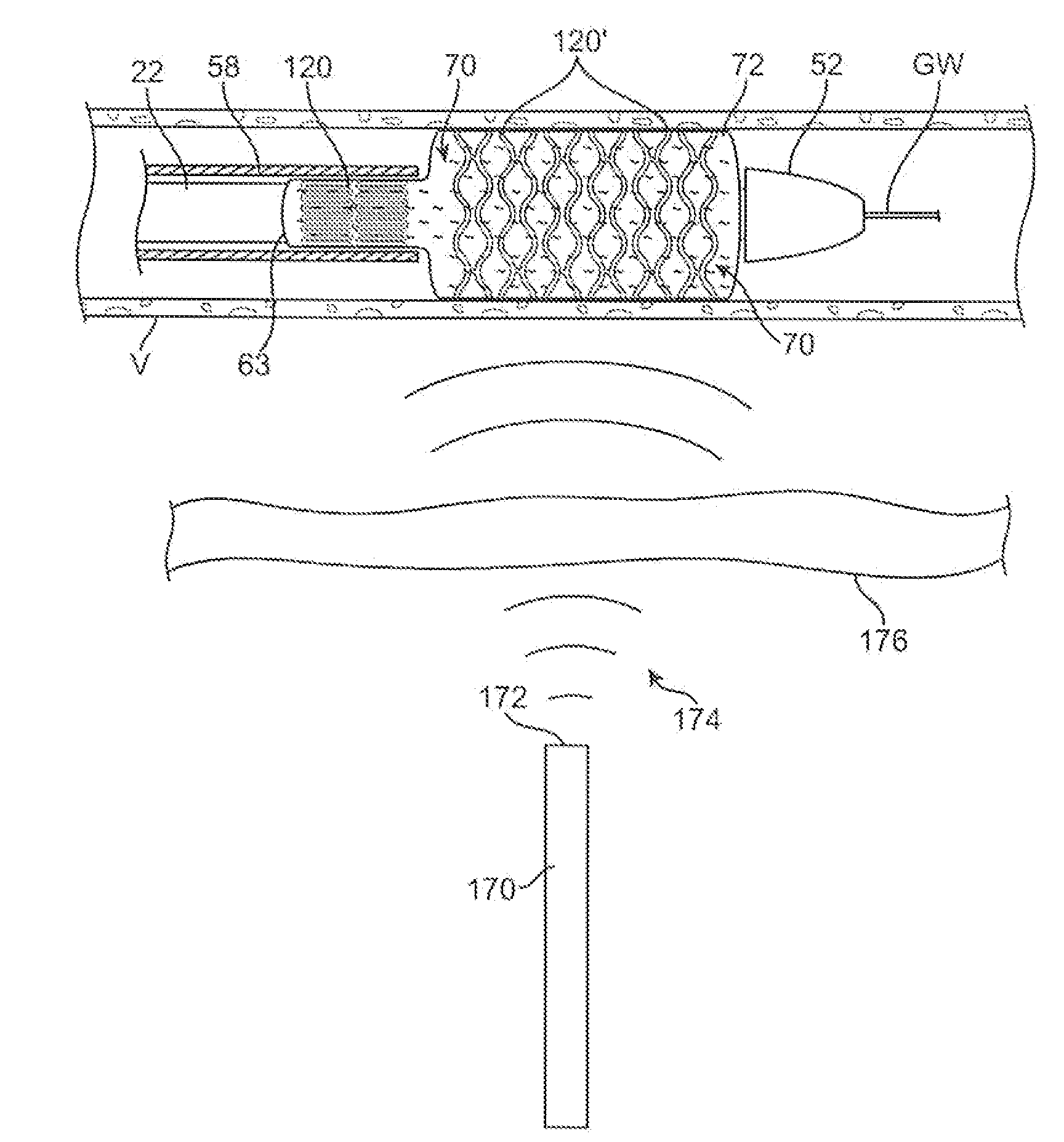
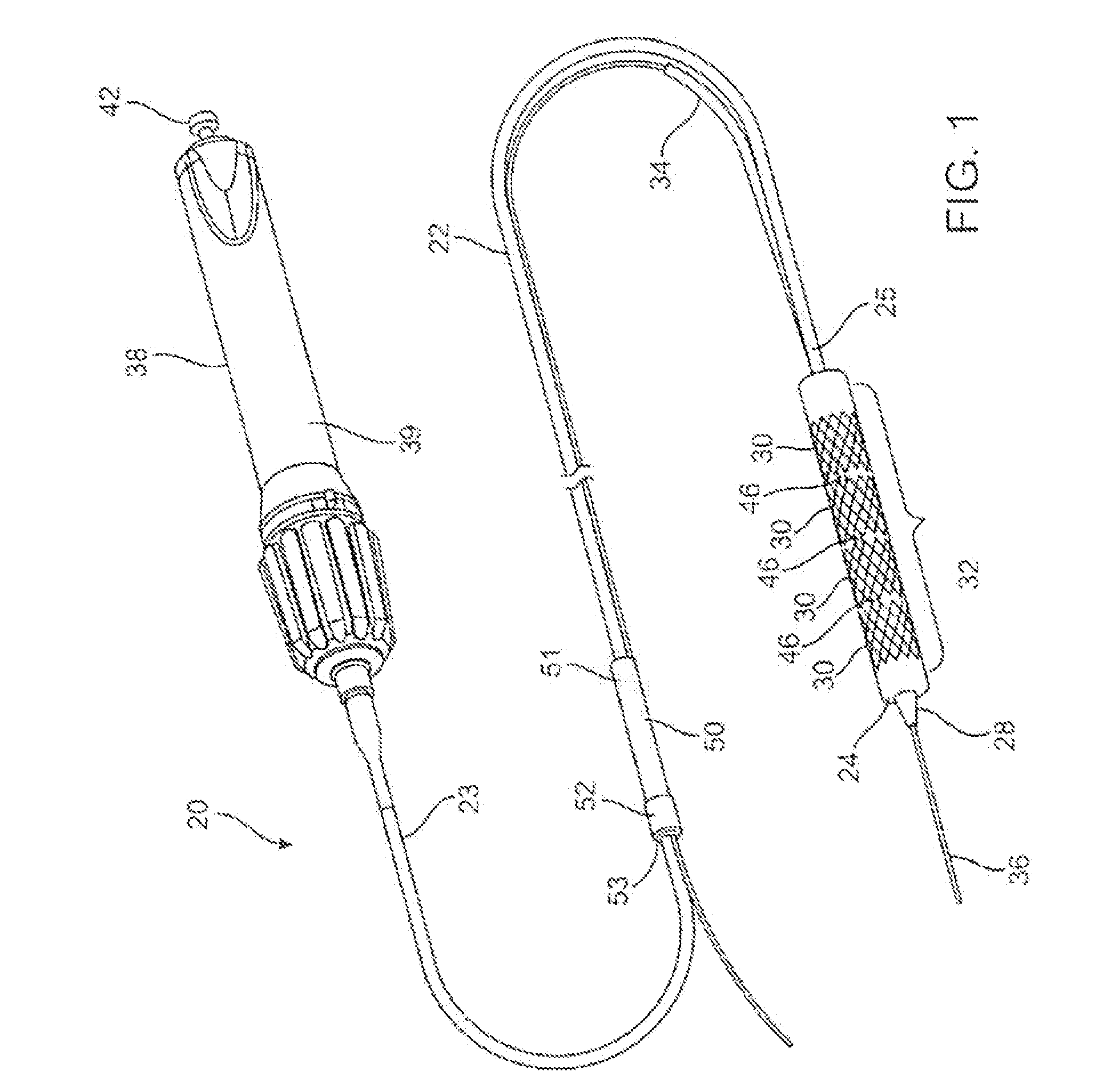
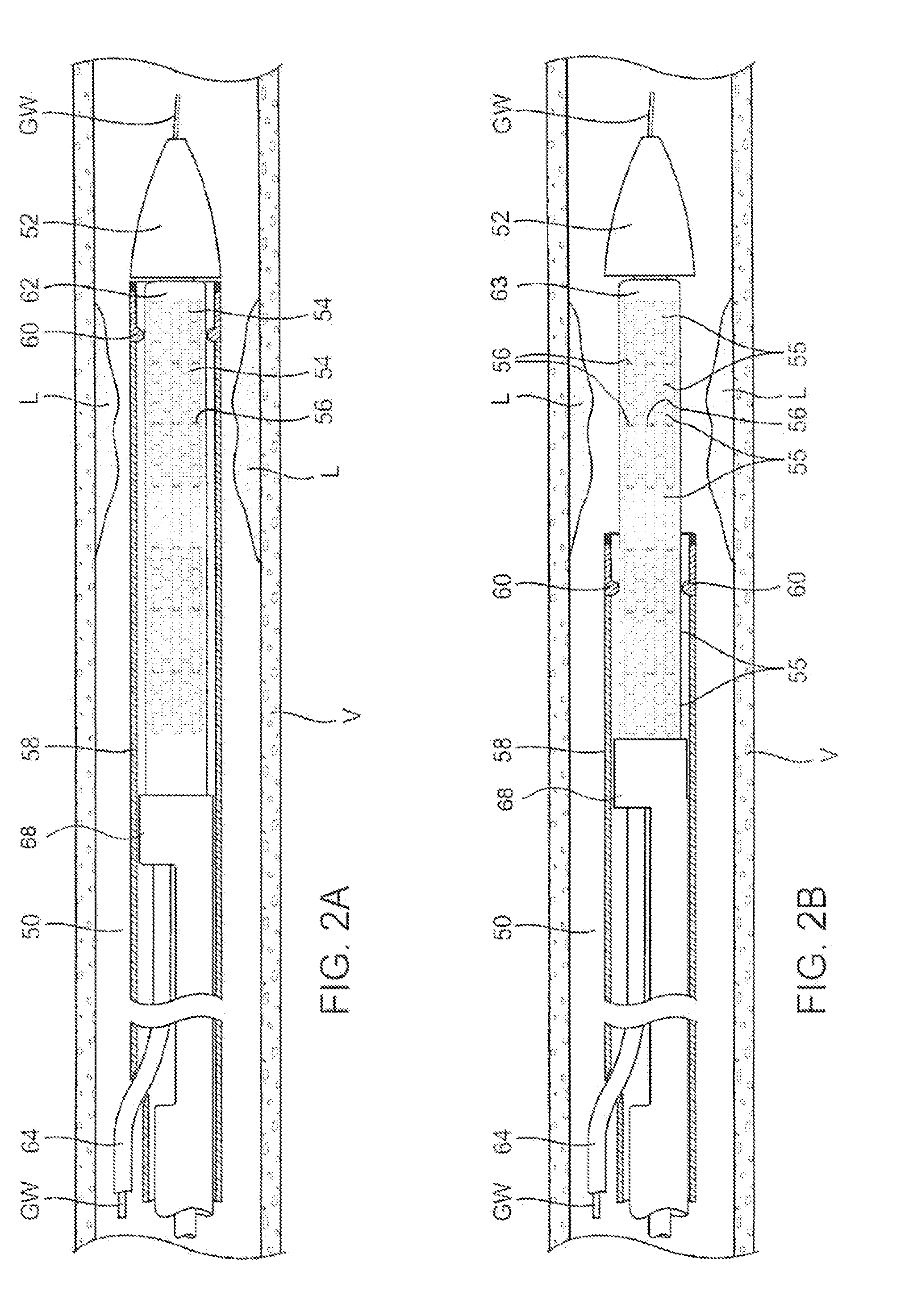
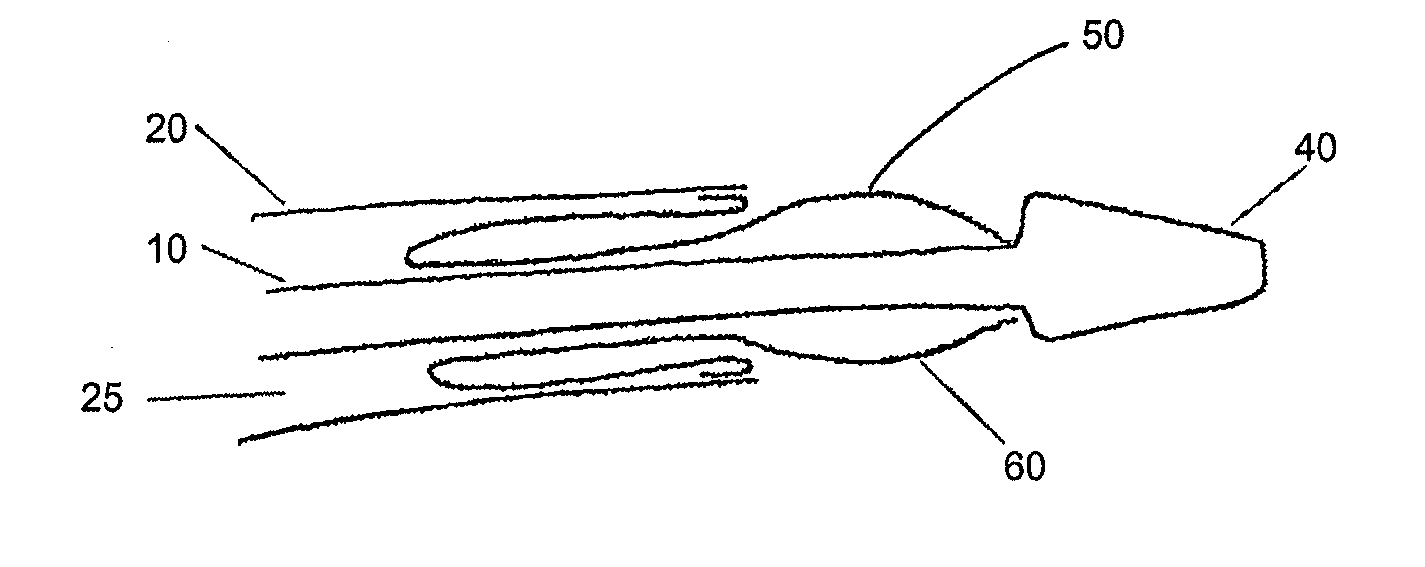
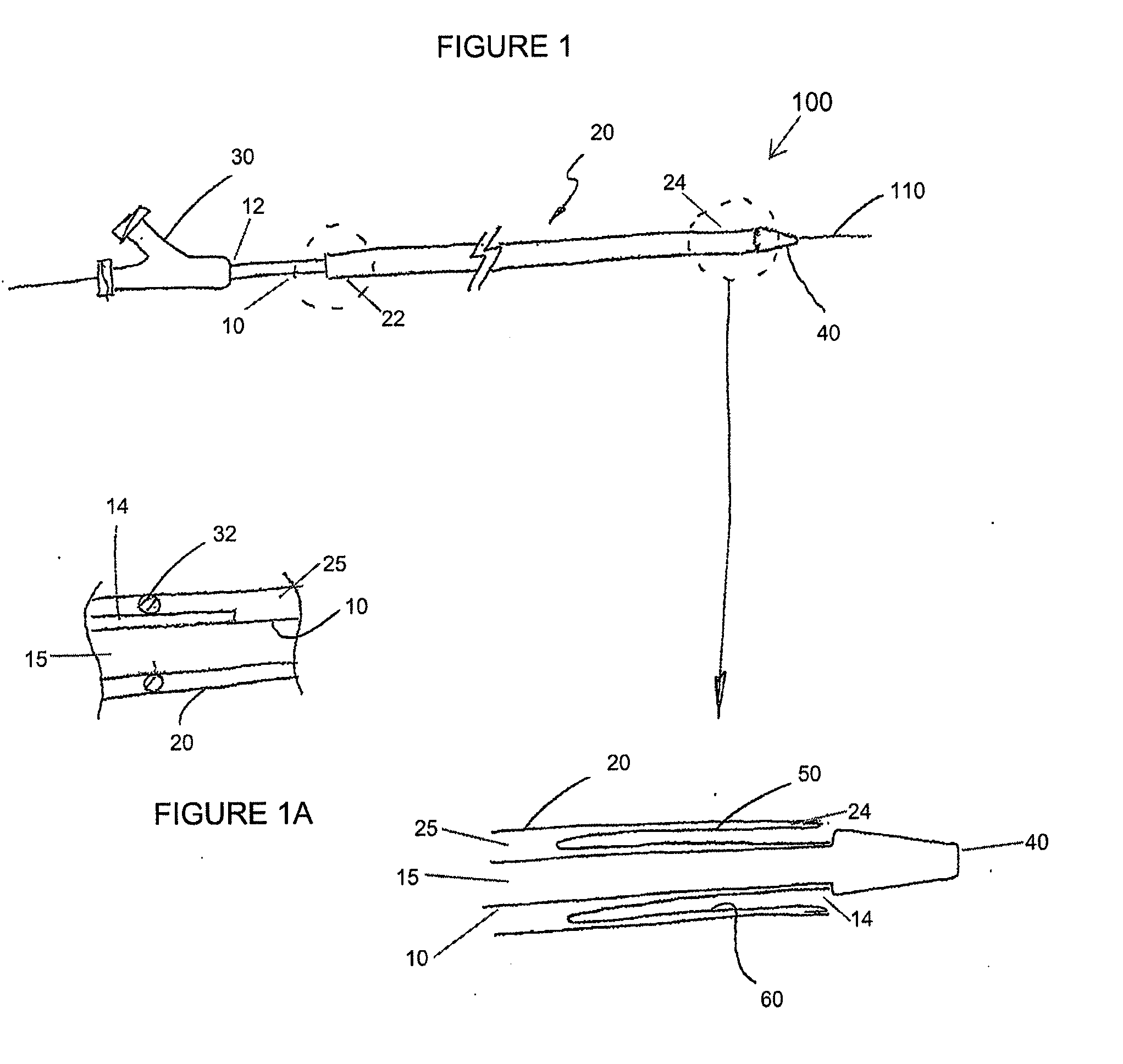
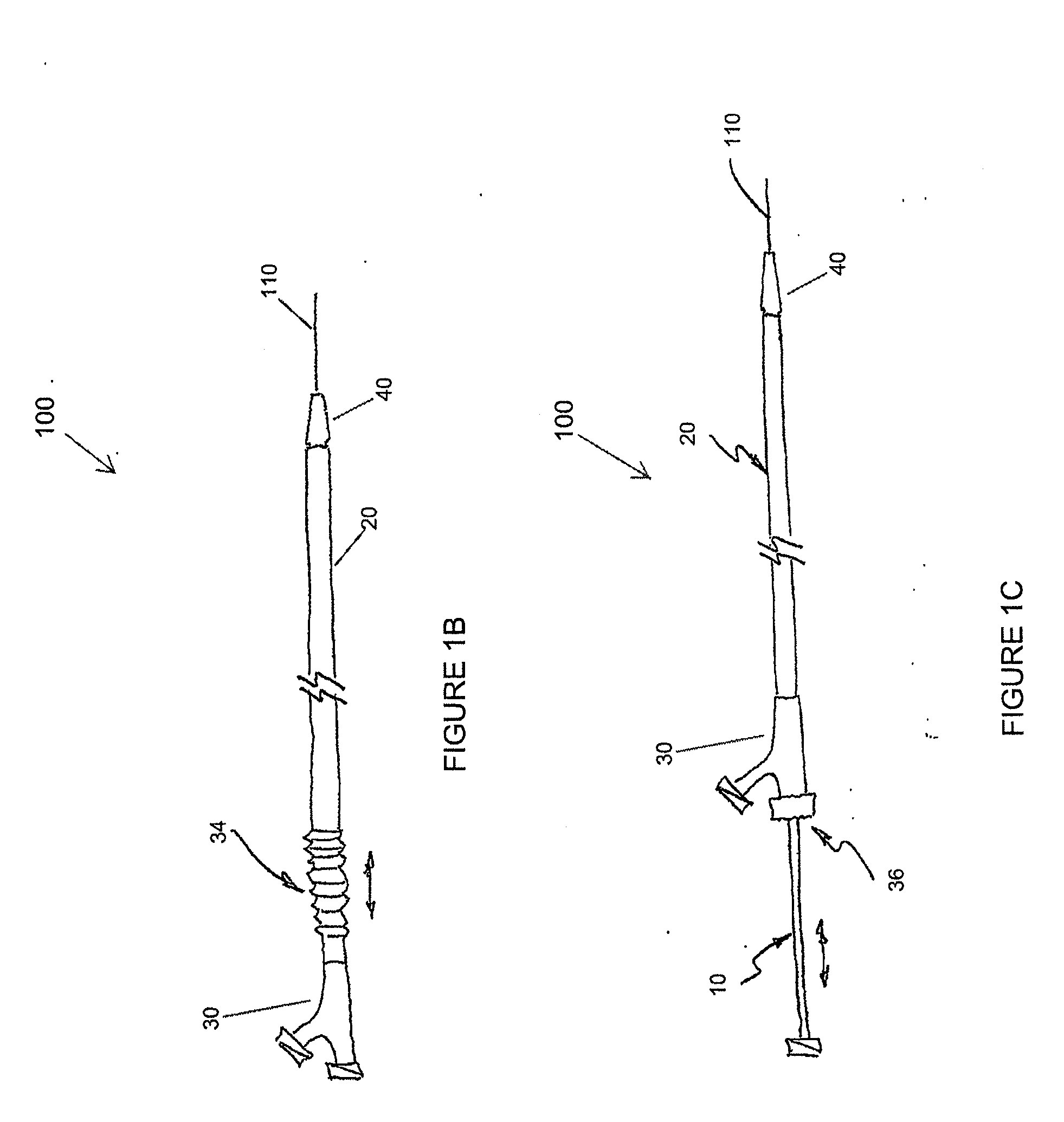
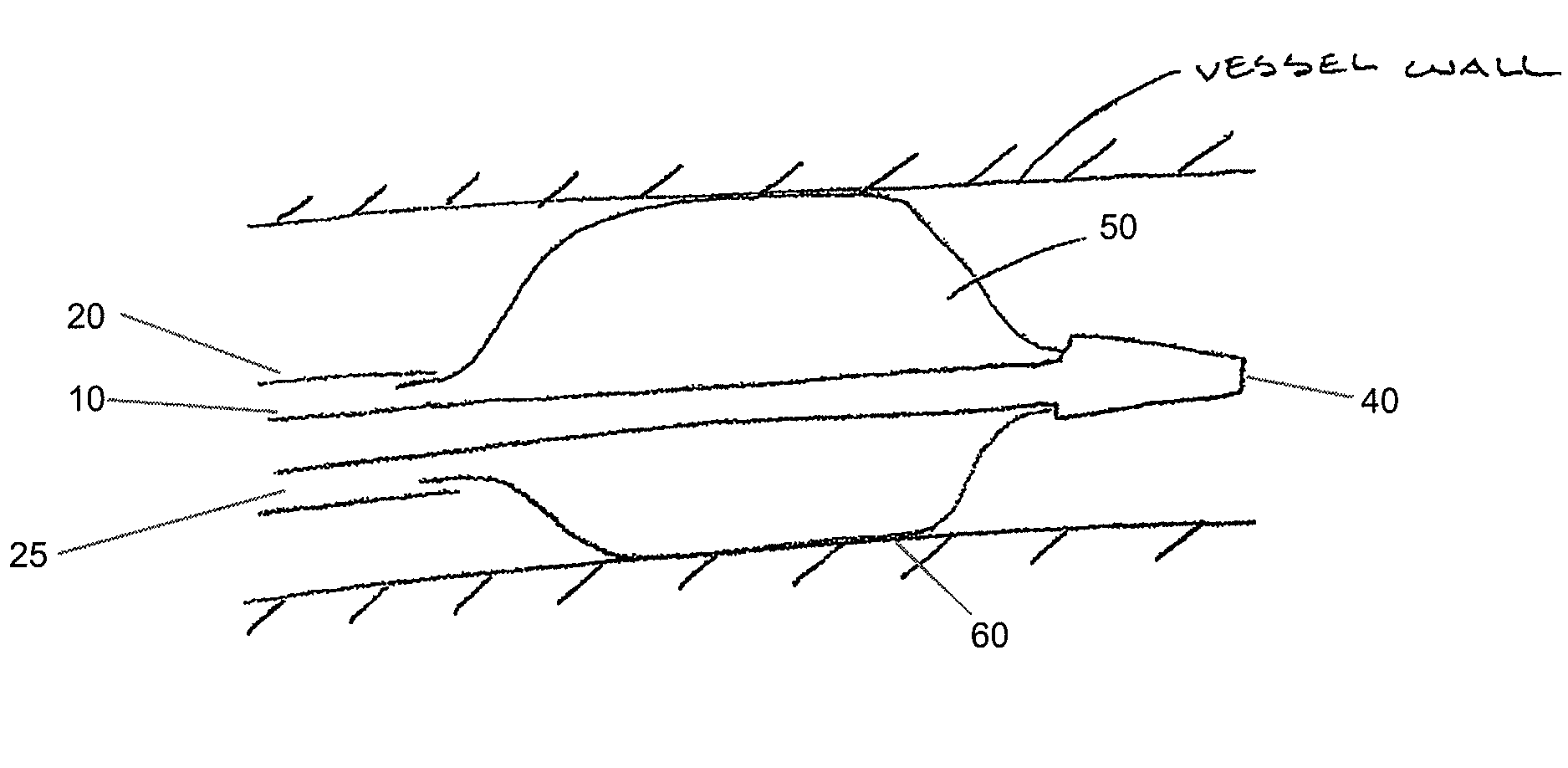
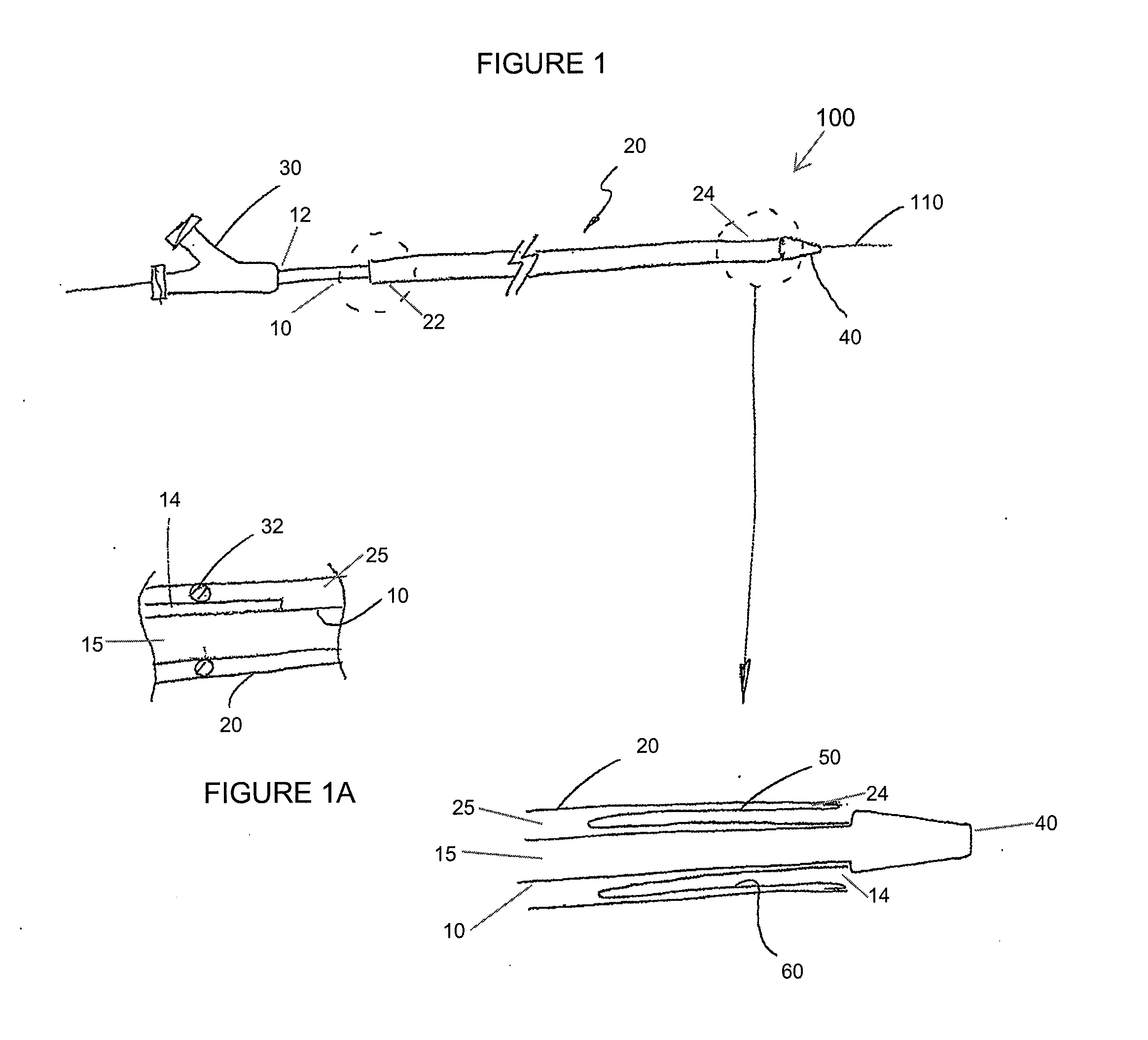
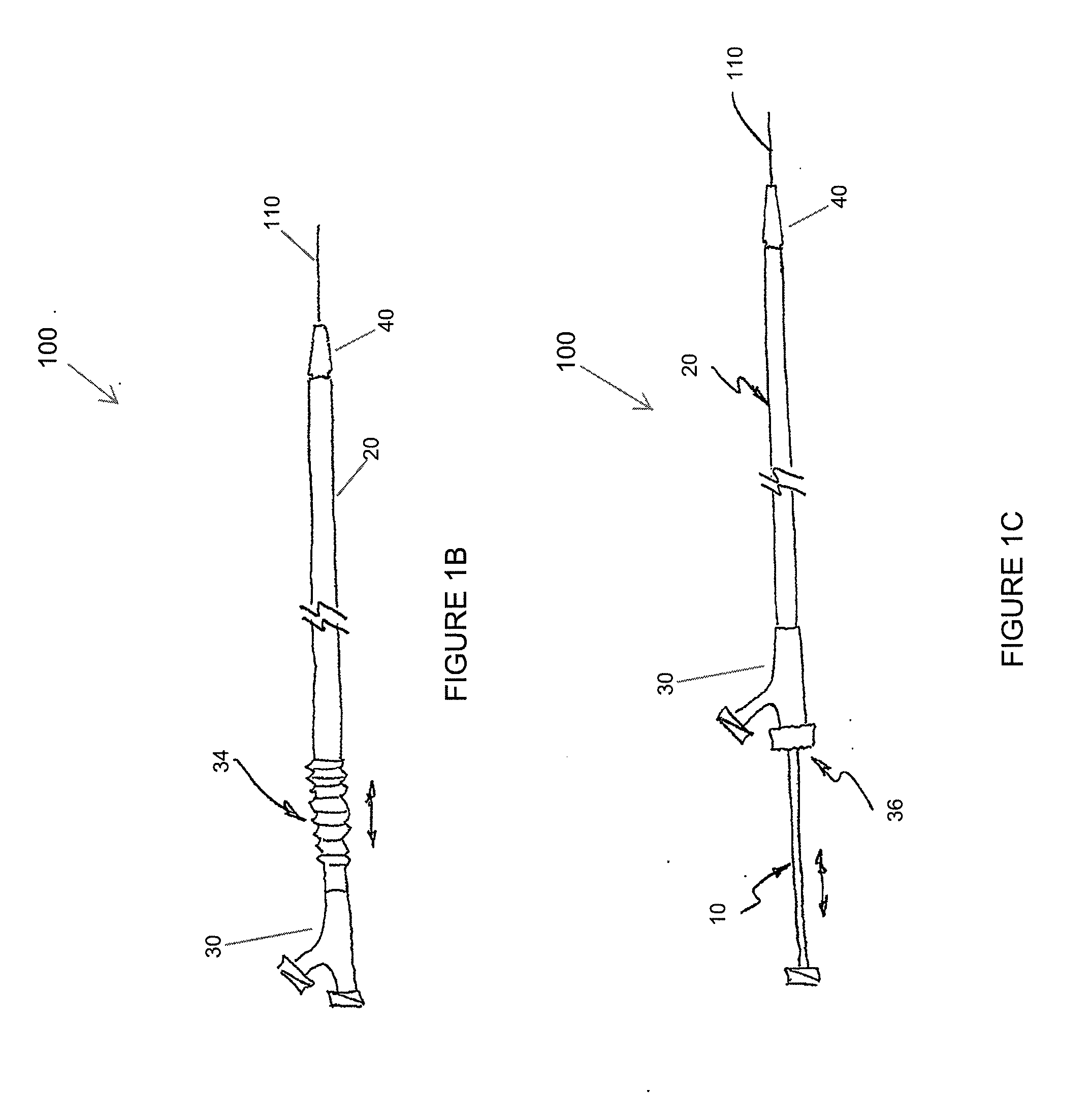
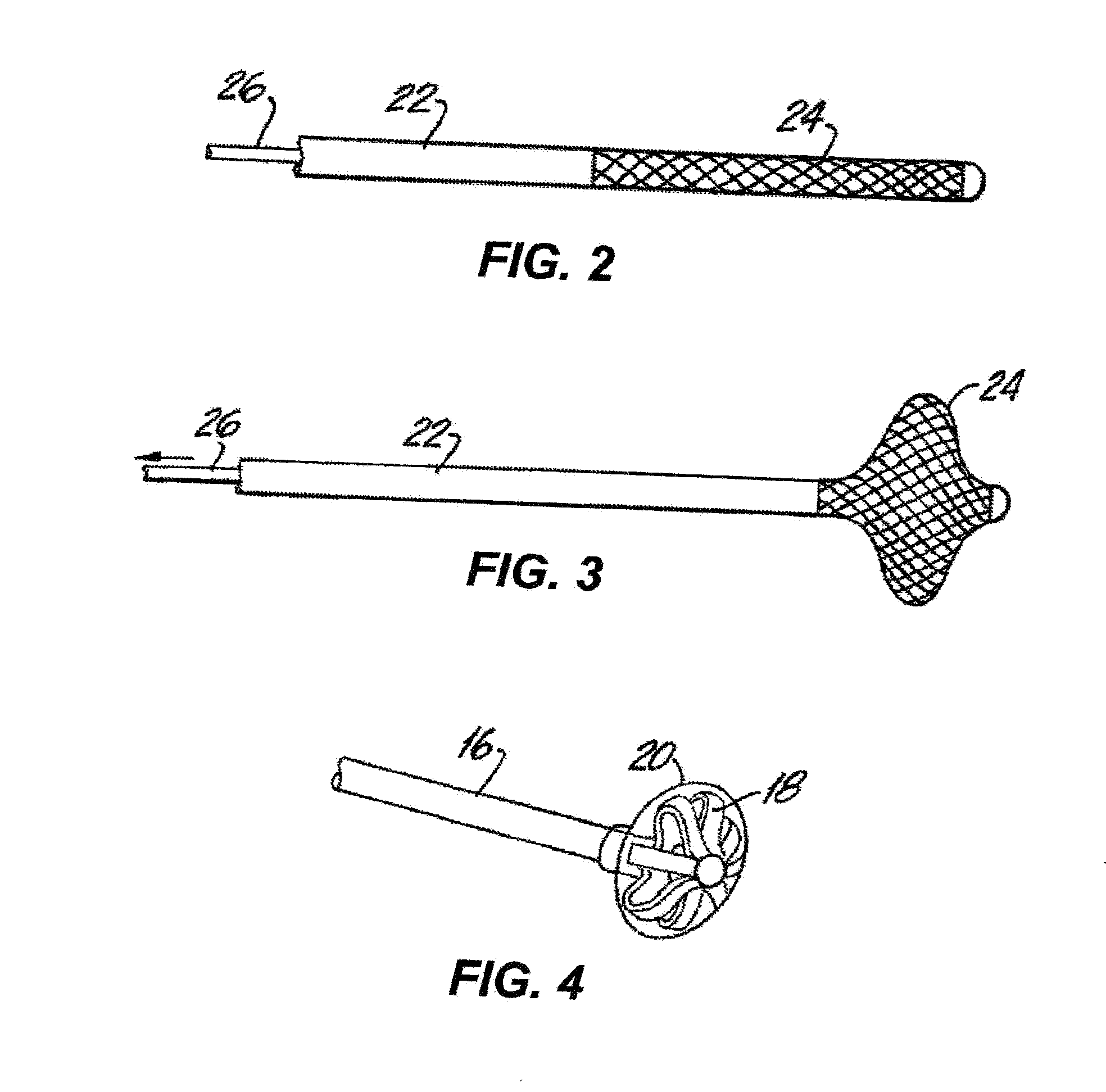
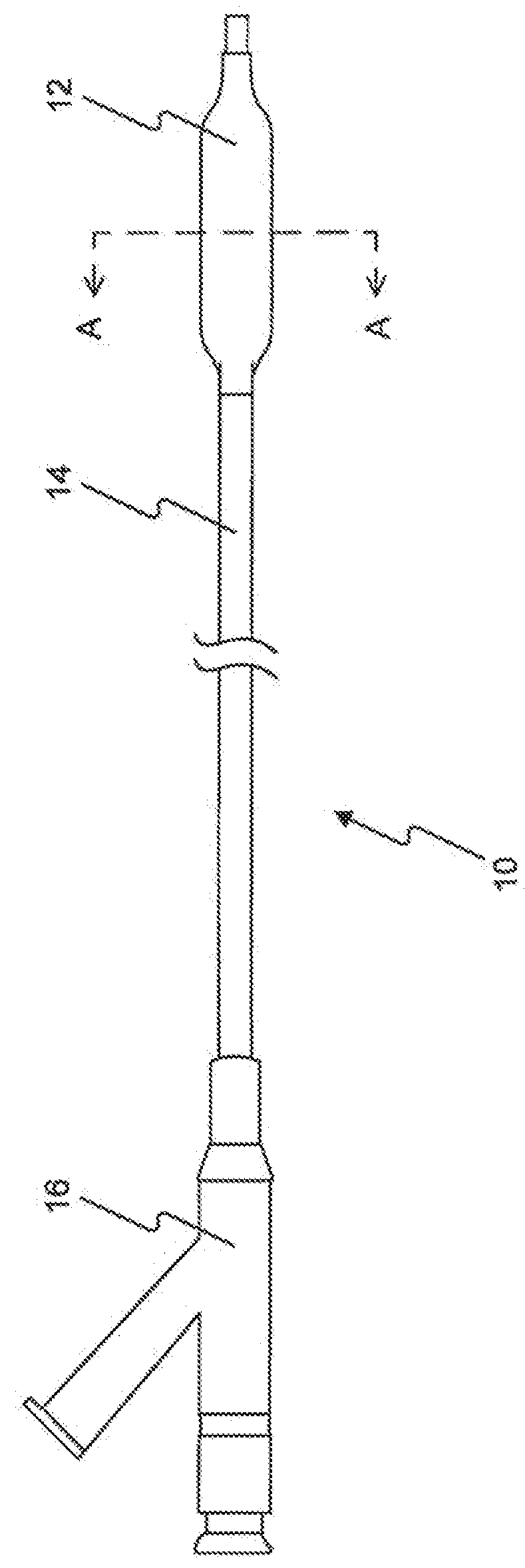
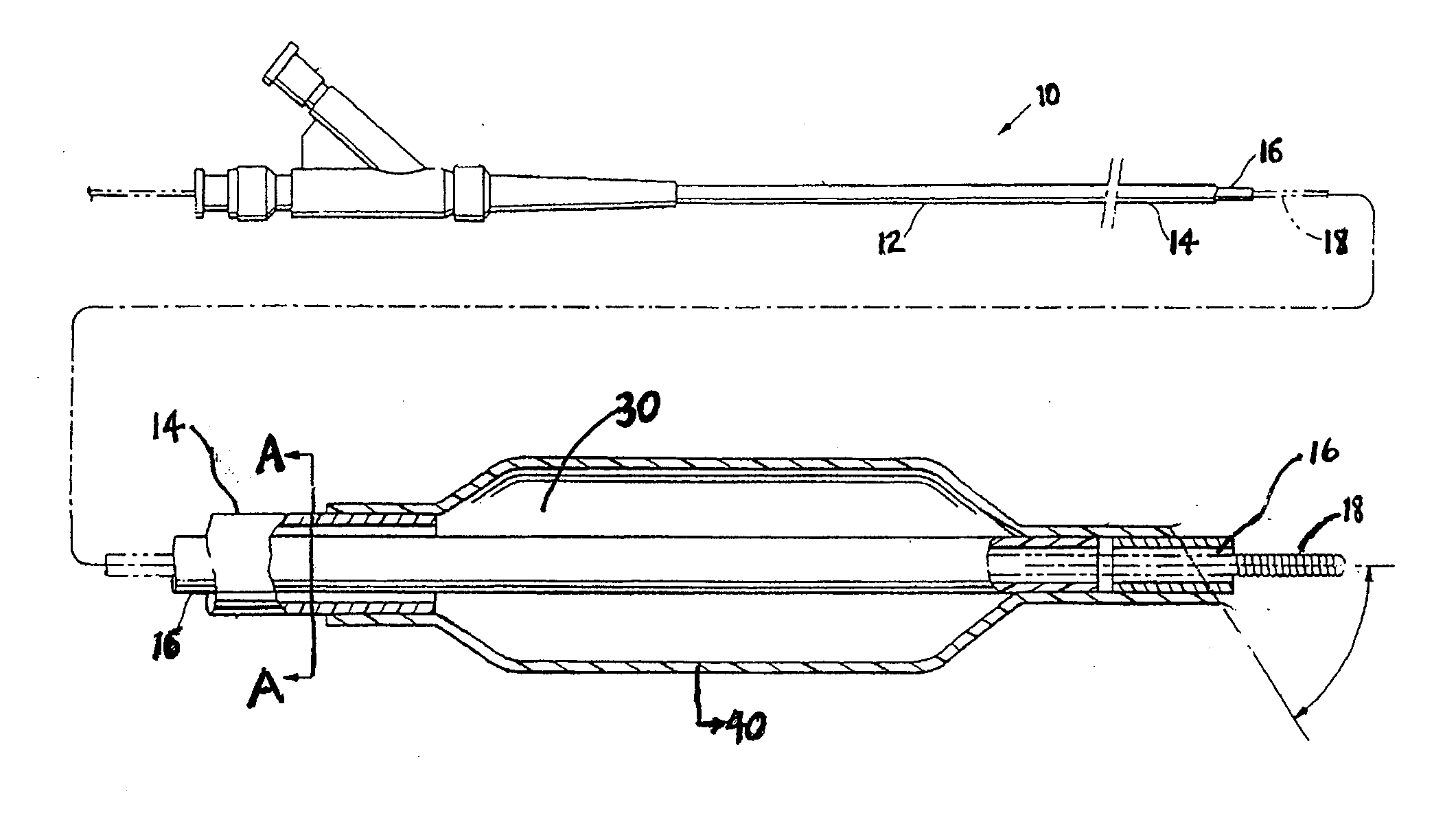
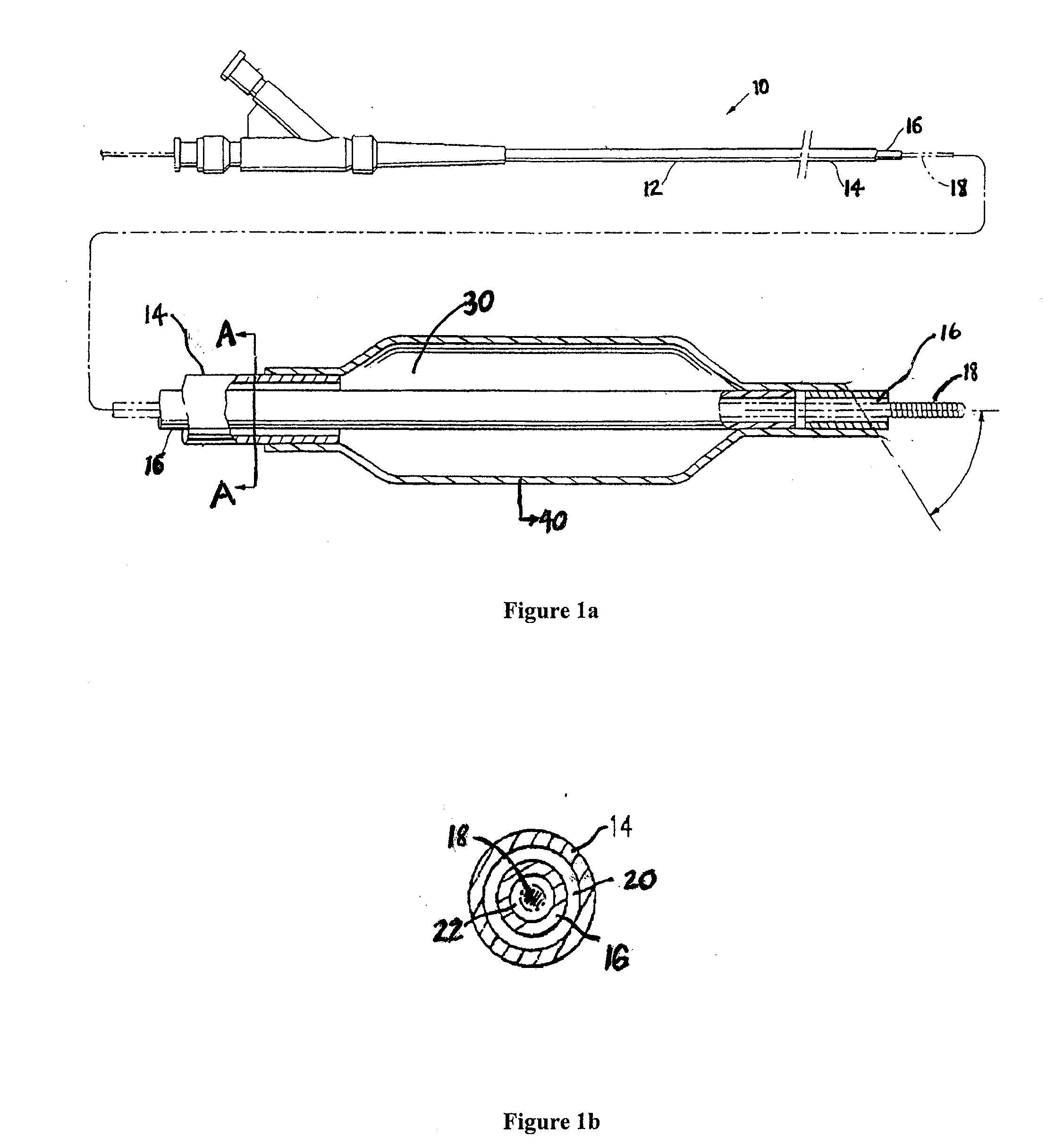
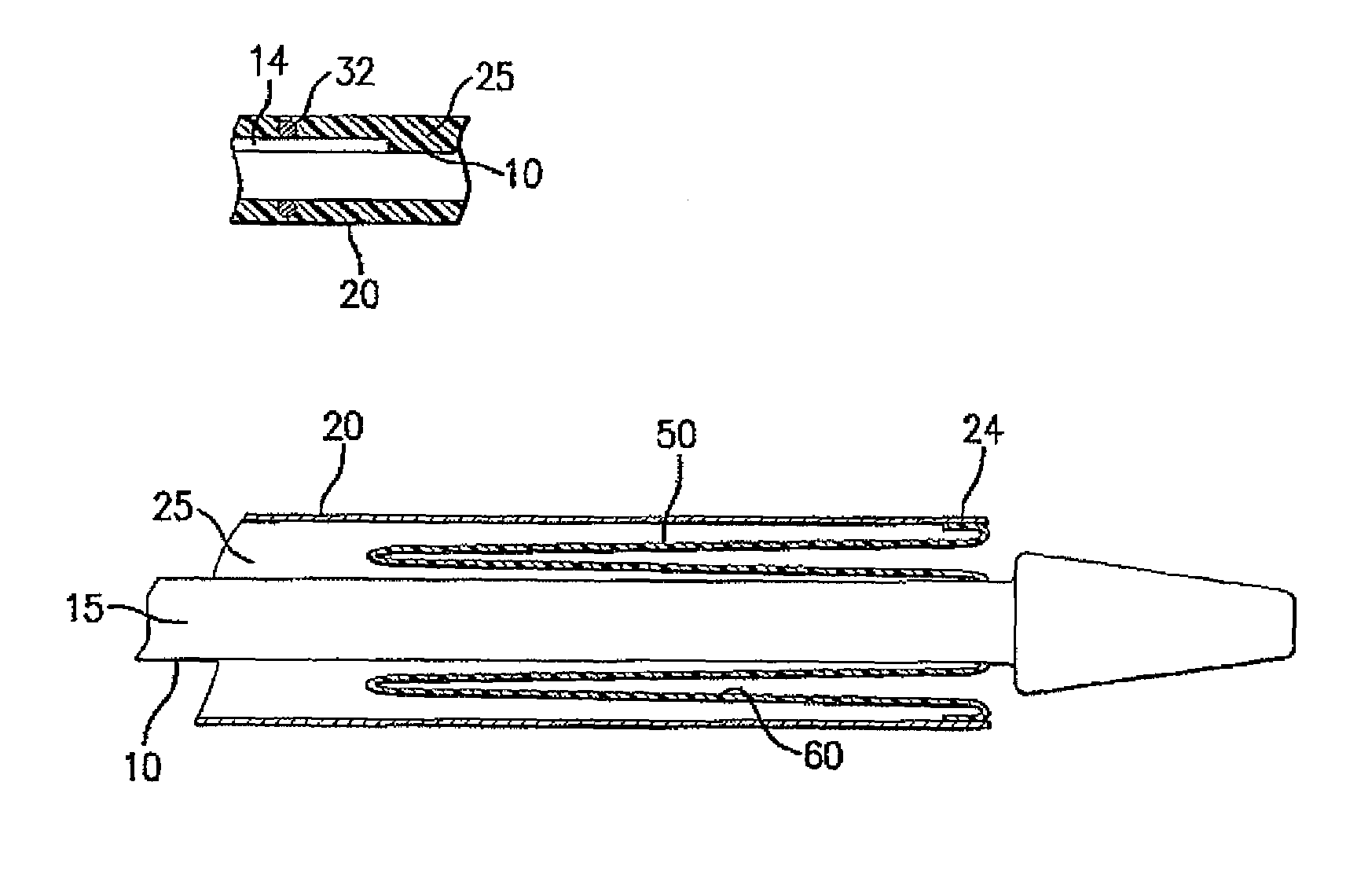
A drug or agent coated balloon having a length which is adjustable in vivo is described herein. The balloon is configured to be coated or coatable with one or more drugs where the coating may be applied prior to advancement into a patient body or prior to balloon expansion within the patient body. The length of the expandable portion of the balloon is adjustable to approximate a length of the tissue region to be treated. Moreover, the drug-coated balloon may be used alone or it may be utilized to deploy luminal prostheses having one or more linked or otherwise coupled segments.
Owner:JW MEDICAL SYSTEMS LTD
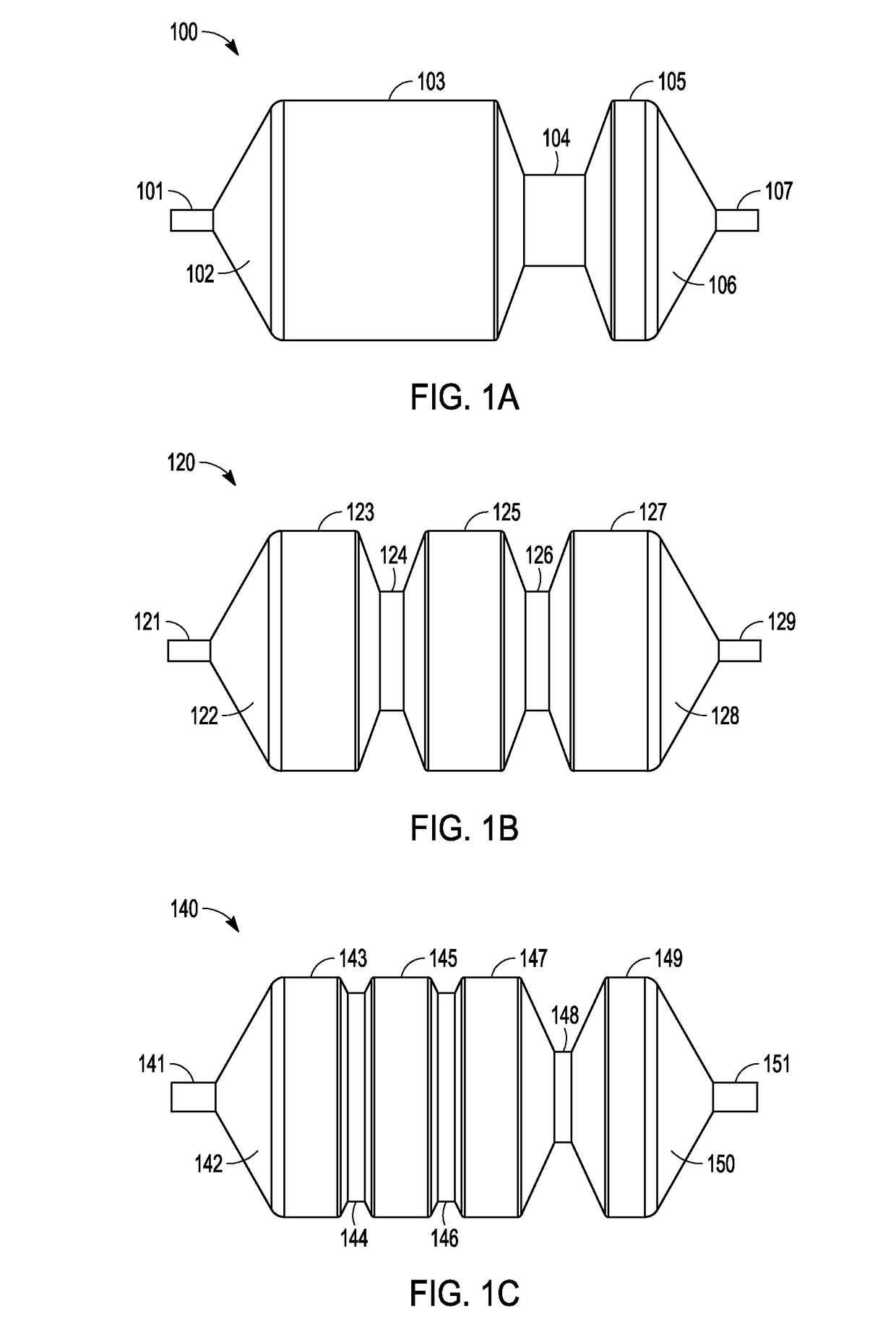
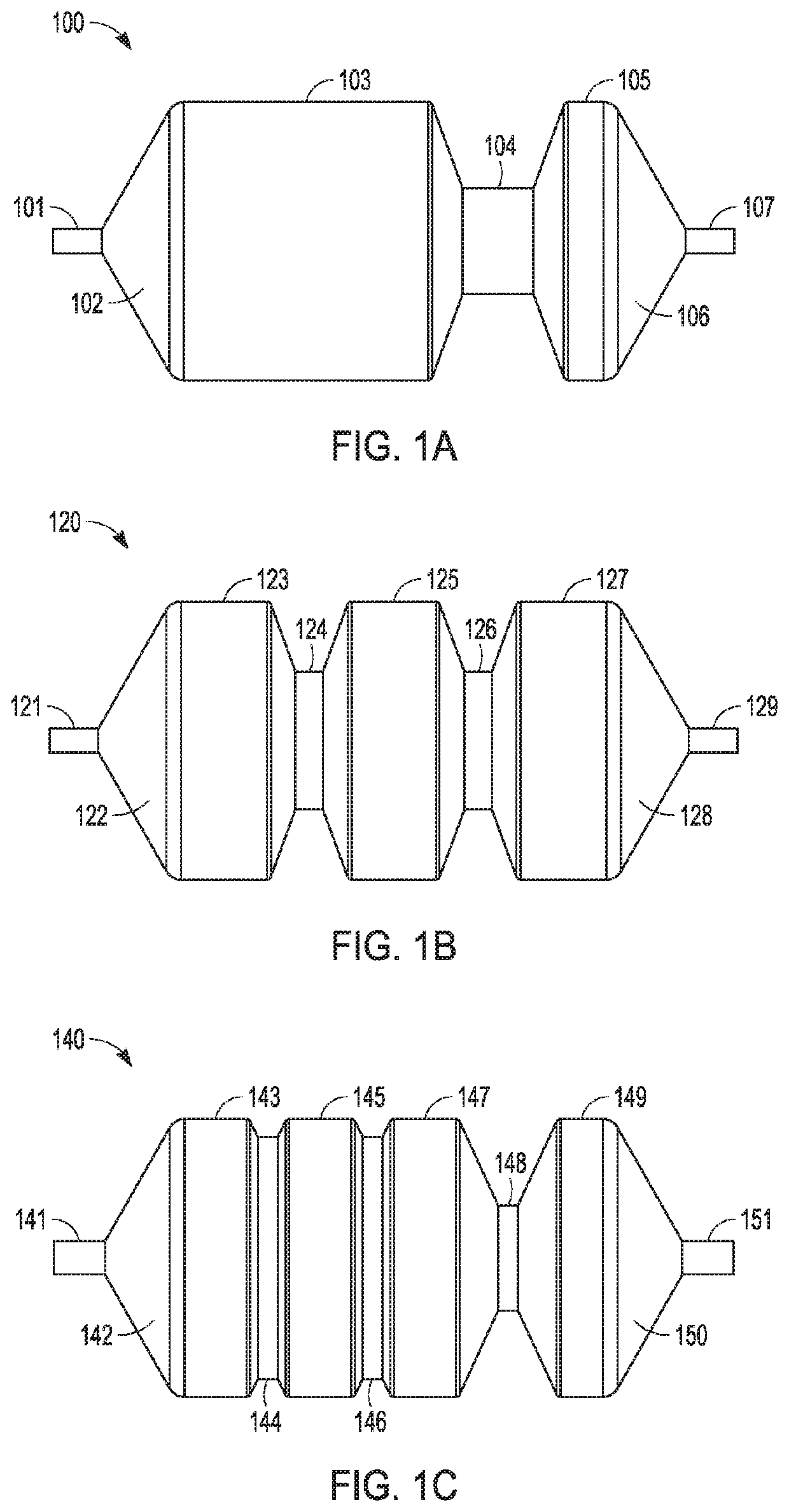
Coatings with tunable molecular architecture for drug-coated balloon
InactiveUS20110143014A1Low elastic modulusLow release rateStentsBalloon catheterDissolutionNuclear medicine
A drug delivery balloon is provided, the a balloon having an outer surface, and a tunable coating disposed on at least a length of the balloon surface. The tunable coating includes a first therapeutic agent and a first excipient, and can include a second therapeutic agent and a second excipient. The first and second therapeutic agents have different dissolution rates during balloon inflation and therefore provide a coating that is tunable.
Owner:ABBOTT CARDIOVASCULAR
Use of Drug Polymorphs to Achieve Controlled Drug Delivery From a Coated Medical Device
When making a medical device having a drug coating thereon, the drug having a plurality of characteristic morphological forms, the manufacturing process is controlled to produce a predetermined ratio of said morphological forms on the device. The process has application to drug coated balloons.
Owner:BOSTON SCI SCIMED INC
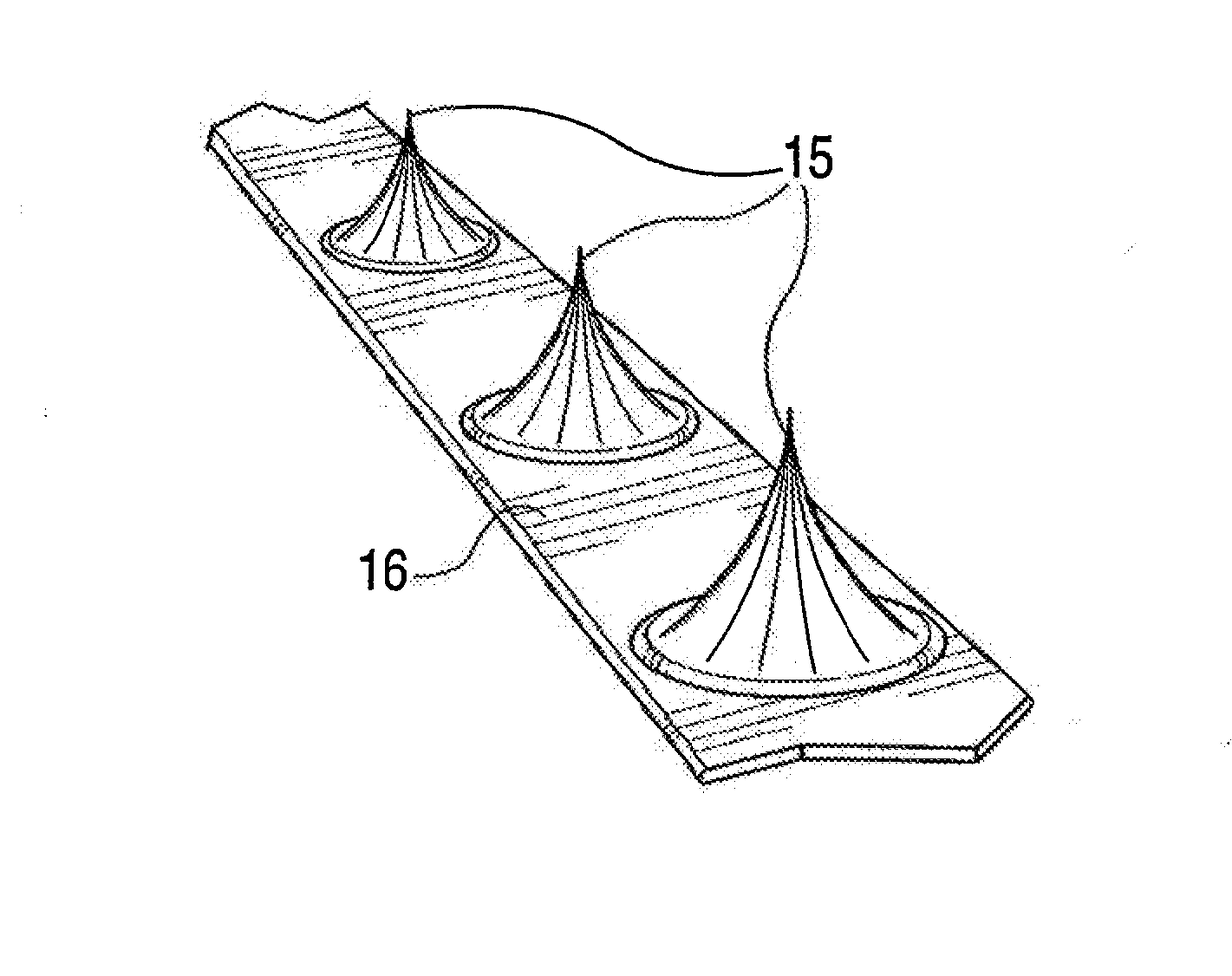
Pre-angioplasty serration of atherosclerotic plaque enabling low-pressure balloon angioplasty and avoidance of stenting
ActiveUS20100042121A1Large caliberSafely and accurately dilated and stretchedBalloon catheterCannulasPercutaneous angioplastyDrug eluting balloon
A device and method for intravascular treatment of atherosclerotic plaque prior to balloon angioplasty which microperforates the plaque with small sharp spikes acting as serrations for forming cleavage lines or planes in the plaque. The spikes may also be used to transport medication into the plaque. The plaque preparation treatment enables subsequent angioplasty to be performed at low balloon pressures of about 4 atmospheres or less, reduces dissections, and avoids injury to the arterial wall. The subsequent angioplasty may be performed with a drug-eluting balloon (DEB) or drug-coated balloon (DCB). The pre-angioplasty perforation procedure enables more drug to be absorbed during DEB or DCB angioplasty, and makes the need for a stent less likely. Alternatively, any local incidence of plaque dissection after balloon angioplasty may be treated by applying a thin, ring-shaped tack at the dissection site only, rather than applying a stent over the overall plaque site.
Owner:CAGENT VASCULAR INC
Drug coated balloon catheter and pharmacokinetic profile
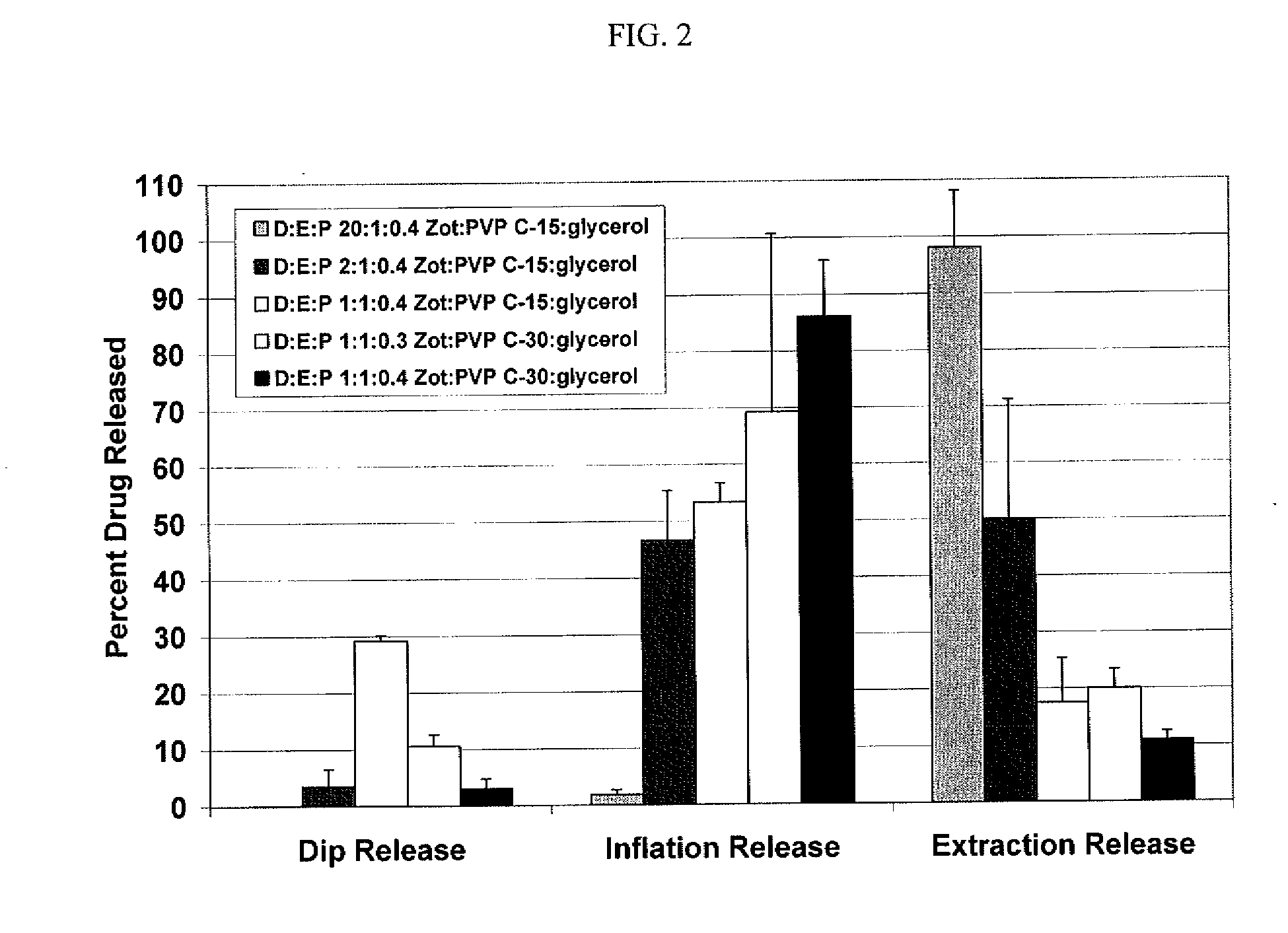
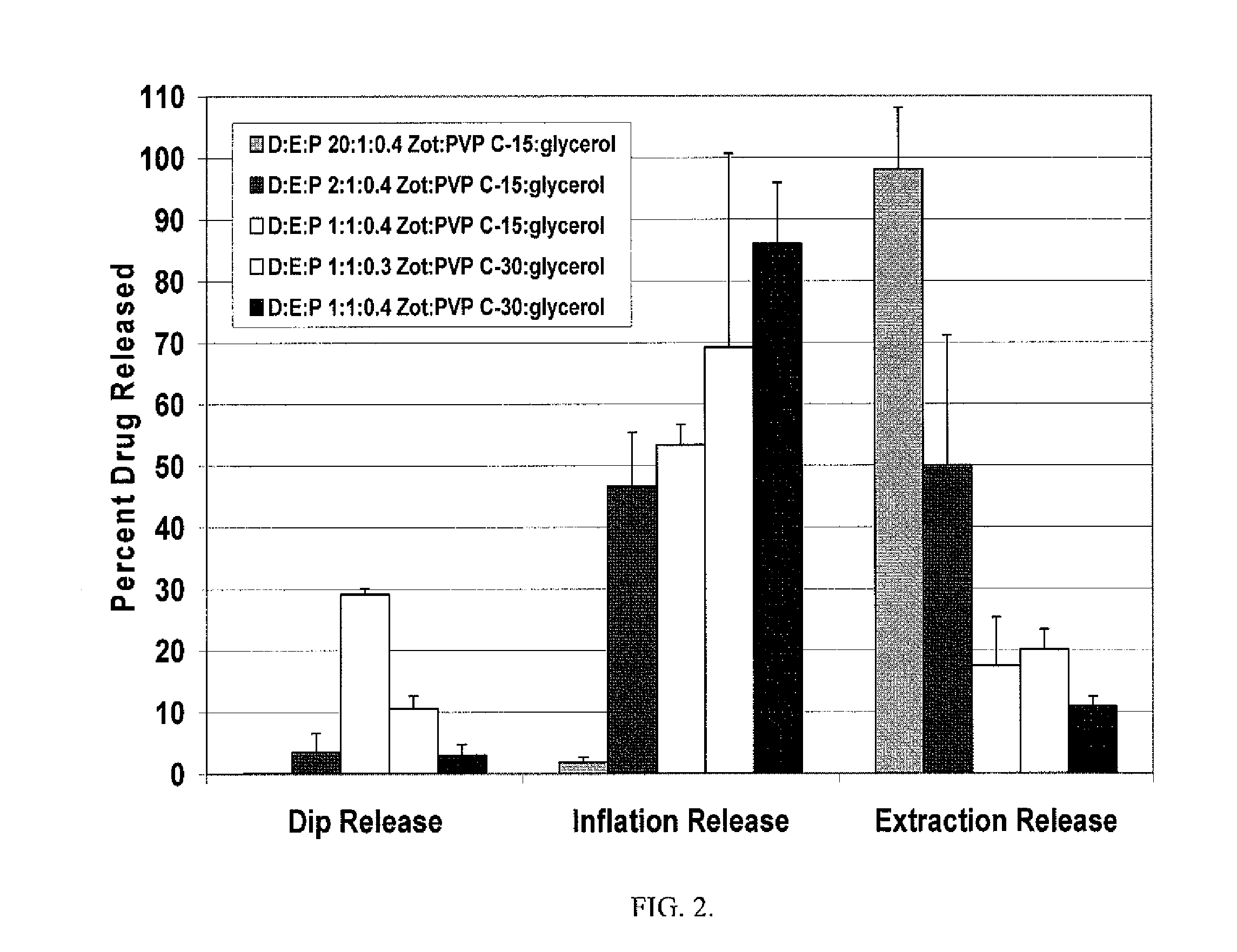
A drug delivery balloon is provided comprising a balloon having a surface, and a coating disposed on at least a portion of the balloon surface, the coating including an cytostatic therapeutic agent, an excipient, and a plasticizer. In accordance with the present subject matter, at least 30% of the coating transfers from the balloon surface within two minutes after inflation of the balloon. Alternatively, at least 30% of the coating transfers from the balloon surface within one minute after inflation. The coating results in an effective pharmacokinetic profile of an cytostatic therapeutic agent in a vasculature or target tissue.
Owner:ABBOTT CARDIOVASCULAR
Hydrophobic therapueutic agent and solid emulsifier coating for drug coated balloon
InactiveUS20110144578A1Improved coating transfer efficiencyPromote absorptionSurgeryGlovesSolubilityMedicine
The disclosed subject matter is directed to a coated medical device such as a balloon or stent and methods of manufacturing the device, where the device has a working length disposed between a distal end and a proximal end thereof; and a coating applied to at least a length of the body. The coating includes a hydrophobic therapeutic agent having a water solubility less than about 15.0 μg / ml and an emulsifier that is a solid at ambient temperature.
Owner:ABBOTT CARDIOVASCULAR
Hydrophilic coatings with tunable composition for drug coated balloon
InactiveUS20110144577A1Minimized drug lossImproved drug recoveryBiocideSurgeryHydrophilic coatingPlasticizer
A tunable coating formulation is described for a drug delivery balloon comprising a therapeutic agent, an excipient and a plasticizer. The tunable coating includes a first therapeutic agent and a first excipient, and can have a second therapeutic agent and a second excipient. The first and second therapeutic agents have different dissolution rates during balloon inflation and therefore provide a coating that is tunable. The plasticizer in the formulation has a weigh ratio of excipient to plasticizer below 1:0.1.
Owner:ABBOTT CARDIOVASCULAR
Drug Coated Balloon Catheter
The present invention relates to balloon catheters for treating a luminal system of a patient. Specifically, the invention relates to catheters having a flexible membrane positioned at a distal portion of the catheter, the flexible membrane retained in a substantially unexposed conformation prior to deployment. Preferably the flexible membrane is capable of delivering a therapeutic agent to a localized environment when deployed to an exposed conformation.
Owner:ABBOTT CARDIOVASCULAR
Drug Coated Balloon Catheter
The present invention relates to balloon catheters for treating a luminal system of a patient. Specifically, the invention relates to catheters having a flexible membrane positioned at a distal portion of the catheter, the flexible membrane retained in a substantially unexposed conformation prior to deployment. Preferably the flexible membrane is capable of delivering a therapeutic agent to a localized environment when deployed to an exposed conformation.
Owner:ABBOTT CARDIOVASCULAR
Drug Coated Balloon Hemostatic Valve Insertion/Balloon Sheath
Introducer sheath for use in inserting a balloon catheter into a patient's vasculature includes a tubular member having an inner diameter, an outer diameter, a proximal end, a distal end, and a length therebetween, the inner diameter being sized to receive a catheter shaft having an attached expandable member in a deflated condition. The inner diameter can be varied from a first inner diameter at the proximal end and a second inner diameter at the distal end, the first inner diameter being greater than the second inner diameter. A method of inserting a balloon catheter into the vasculature of a patient is also disclosed. Additionally, a balloon catheter kit including a catheter and an introducer sheath is disclosed.
Owner:ABBOTT CARDIOVASCULAR
Temporary Vascular Scaffold and Scoring Device
ActiveUS20140142598A1Avoid excessive injuryAvoid complicationsStentsBalloon catheterMedical deviceBlood vessel
Devices and methods for treating a target site in a body lumen are provided. A medical device includes a stent-like structure including a plurality of scoring and non-scoring filaments interwoven with one another. Generally, the stent-like structure will have more non-scoring filaments than scoring filaments to provide greater structural support and to focus the scoring forces on only a few select areas. The stent-like structure is expanded within the target site to score the target site and to provide temporary structural support while the target site is infused with a therapeutic agent. Such therapeutic agent infusion occurs with the use of a drug eluting or drug coated balloon disposed within the stent-like structure or by occluding the target site and introducing a drug into the occluded target site to sit for a period of time.
Owner:NFINIUM VASCULAR TECH
Drug Coated Balloon Catheters for Nonvascular Strictures
ActiveUS20150273117A1Facilitated releasePromote absorptionBalloon catheterAntipyreticEsophageal stenosisBiliary tract
Embodiments of the present invention provide a method for treatment of nonvascular body lumen strictures such as benign prostatic hyperplasia (BPH), urethral strictures, ureteral strictures, prostate cancer, esophageal strictures, sinus strictures, biliary tract strictures, asthma and chronic obstructive pulmonary disease (COPD). The method involves delivering, preferably via drug coated balloon catheters, of anti-inflammatory and anti-proliferative drugs (rapamycin or paclitaxel and their analogues) and one or more additives.
Owner:UROTRONIC
Drug coated balloon catheters for nonvascular strictures
ActiveUS20180104383A1Facilitated releaseImprove permeabilityOrganic active ingredientsBalloon catheterBiliary tractObstructive Pulmonary Diseases
Embodiments of the present invention provide a method of treating a stricture in a nonvascular body lumen such as urethral strictures, benign prostatic hyperplasia (BPH) strictures, ureteral strictures, esophageal strictures, sinus strictures, and biliary tract strictures. Embodiments of the present invention provide a method for treating at least one of benign prostatic hyperplasia (BPH), prostate cancer, asthma, and chronic obstructive pulmonary disease (COPD). The method can include delivering, for example, via drug coated balloon catheters, anti-inflammatory and anti-proliferative drugs (e.g., rapamycin, paclitaxel, and their analogues) and one or more additives.
Owner:UROTRONIC
Drug-coated balloon catheters for body lumens
ActiveUS20190015639A1Avoid bleedingInhibit migrationBalloon catheterMedical devicesWater solubleBalloon catheter
Various embodiments disclosed relate to drug-coated balloon catheters for treating strictures in body lumens and methods of using the same. A drug-coated balloon catheter for delivering a therapeutic agent to a target site of a body lumen stricture includes an elongated balloon having a main diameter. The balloon catheter includes a coating layer overlying an exterior surface of the balloon. The coating layer includes one or more water-soluble additives and an initial drug load of a therapeutic agent.
Owner:UROTRONIC
Drug-coated balloon catheters for body lumens
ActiveUS20190015640A1Avoid bleedingInhibit migrationBalloon catheterMedical devicesWater solubleBalloon catheter
Various embodiments disclosed relate to drug-coated balloon catheters for treating strictures in body lumens and methods of using the same. A drug-coated balloon catheter for delivering a therapeutic agent to a target site of a body lumen stricture includes an elongated balloon having a main diameter. The balloon catheter includes a coating layer overlying an exterior surface of the balloon. The coating layer includes one or more water-soluble additives and an initial drug load of a therapeutic agent.
Owner:UROTRONIC
Balloon catheters for body lumens
ActiveUS20190344053A1Avoid bleedingInhibit migrationOrganic active ingredientsBalloon catheterCoelomWater soluble
Various embodiments disclosed relate to drug-coated balloon catheters for treating strictures in body lumens and methods of using the same. A drug-coated balloon catheter for delivering a therapeutic agent to a target site of a body lumen stricture includes an elongated balloon having a main diameter. The balloon catheter includes a coating layer overlying an exterior surface of the balloon. The coating layer includes one or more water-soluble additives and an initial drug load of a therapeutic agent.
Owner:UROTRONIC
Drug-coated balloon catheter
InactiveCN106267377ASmall particle sizeImprove absorption rateSurgeryCatheterMass ratioTarget tissue
The invention relates to a drug-coated balloon catheter. The drug-coated balloon catheter comprises a balloon and a drug coating on the surface of the balloon. The drug coating comprises an active drug and a carrier. The active drug is paclitaxel, rapamycin, a paclitaxel derivative or a rapamycin derivative. The carrier comprises an organic acid salt and polyol. A mass ratio of the active drug to the carrier in the drug coating is 0.5-49. A mass ratio of the organic acid salt to polyol is (3-50): 1. The organic acid salt and polyol in the drug coating produce synergism, drug particles have small particle sizes, premature release of the drug before displacement of the balloon catheter to a target point is prevented, balloon surface drug fast release is promoted, drug absorption by target tissue is promoted, a drug loss is reduced in a conveying process, particle falling is reduced, coating safety is improved and good drug transshipment effects are obtained.
Owner:LIFETECH SCIENTIFIC (SHENZHEN) CO LTD
Coating Process for Drug Delivery Balloons Using Heat-Induced Rewrap Memory
A method of producing a drug coated balloon that comprises the steps of: subjecting a balloon catheter with a folded and wrapped balloon thereon to a pre-annealing step to induce a fold / wrap memory in the resulting pre-annealed balloon; unfolding the pre-annealed balloon sufficiently to expose the full circumferential surface of the balloon by application of an inflation pressure that retains said fold / wrap memory; applying a drug coating formulation to the unfolded balloon surface; releasing pressure to relax the balloon and induce creasing along fold memory; and evacuating the balloon slowly to induce refolding and rewrapping of the balloon. The method overcomes the need to use a folding apparatus to fold and wrap a drug coated balloon.
Owner:BOSTON SCI SCIMED INC
Drug coated balloon catheters for nonvascular strictures
ActiveUS20190167854A1Avoid dependenceAvoid disadvantagesAntipyreticAnalgesicsBiliary tractObstructive Pulmonary Diseases
Embodiments of the present invention provide a method for treatment of nonvascular body lumen strictures such as benign prostatic hyperplasia (BPH), urethral strictures, ureteral strictures, prostate cancer, esophageal strictures, sinus strictures, biliary tract strictures, asthma and chronic obstructive pulmonary disease (COPD). The method involves delivering, preferably via drug coated balloon catheters, of anti-inflammatory and anti-proliferative drugs (rapamycin or paclitaxel and their analogues) and one or more additives.
Owner:UROTRONIC
Drug coated balloon catheter
The present invention relates to balloon catheters for treating a luminal system of a patient. Specifically, the invention relates to catheters having a flexible membrane positioned at a distal portion of the catheter, the flexible membrane retained in a substantially unexposed conformation prior to deployment. Preferably the flexible membrane is capable of delivering a therapeutic agent to a localized environment when deployed to an exposed conformation.
Owner:ABBOTT CARDIOVASCULAR
Drug coated balloon catheter
The present invention relates to balloon catheters for treating a luminal system of a patient. Specifically, the invention relates to catheters having a flexible membrane positioned at a distal portion of the catheter, the flexible membrane retained in a substantially unexposed conformation prior to deployment. Preferably the flexible membrane is capable of delivering a therapeutic agent to a localized environment when deployed to an exposed conformation.
Owner:ABBOTT CARDIOVASCULAR
Drug coated balloon catheters for nonvascular strictures
ActiveUS10888640B2Promote absorptionAvoid dependenceOrganic active ingredientsBalloon catheterBile duct stricturesVascular body
Owner:UROTRONIC
System and method for plaque serration
ActiveUS20170106174A1Safely and accurately dilated and stretchedDilated more evenly and smoothlyBalloon catheterMicroneedlesDrug eluting balloonPars plana
A device and method for intravascular treatment of atherosclerotic plaque prior to balloon angioplasty which microperforates the plaque with small sharp spikes acting as serrations for forming cleavage lines or planes in the plaque. The spikes may also be used to transport medication into the plaque. The plaque preparation treatment enables subsequent angioplasty to be performed at low balloon pressures of about 4 atmospheres or less, reduces dissections, and avoids injury to the arterial wall. The subsequent angioplasty may be performed with a drug-eluting balloon (DEB) or drug-coated balloon (DCB). The pre-angioplasty perforation procedure enables more drug to be absorbed during DEB or DCB angioplasty, and makes the need for a stent less likely. Alternatively, any local incidence of plaque dissection after balloon angioplasty may be treated by applying a thin, ring-shaped tack at the dissection site only, rather than applying a stent over the overall plaque site.
Owner:CAGENT VASCULAR INC
Treatment Of Diabetic Patients With A Drug Eluting Stent And A Drug Coated Balloon
Embodiments of the present invention include methods for the treatment, prevention, or amelioration of vascular disease and / or disorder in diabetic patients. The methods include implantation of a stent including a drug, and the use of a drug coated balloon. The DES may be a DES having a metal body and a coating including the drug, or a bioabsorbable stent with drug in the body of the stent, in a coating on the stent, or both in the body of the stent and in a coating on the stent.
Owner:ABBOTT CARDIOVASCULAR
Drug coated balloon catheter and pharmacokinetic profile
A drug delivery balloon is provided comprising a balloon having a surface, and a coating disposed on at least a portion of the balloon surface, the coating including an cytostatic therapeutic agent, an excipient, and a plasticizer. In accordance with the present subject matter, at least 30% of the coating transfers from the balloon surface within two minutes after inflation of the balloon. Alternatively, at least 30% of the coating transfers from the balloon surface within one minute after inflation. The coating results in an effective pharmacokinetic profile of an cytostatic therapeutic agent in a vasculature or target tissue.
Owner:ABBOTT CARDIOVASCULAR
Coatings with tunable solubility profile for drug-coated balloon
A drug delivery balloon is provided, the a balloon having an outer surface, and a tunable coating disposed on at least a length of the balloon surface. The tunable coating includes a first therapeutic agent and a first excipient, and a second therapeutic agent and a second excipient. The first and second therapeutic agents have different dissolution rates during balloon inflation and therefore provide a coating that is tunable.
Owner:ABBOTT CARDIOVASCULAR
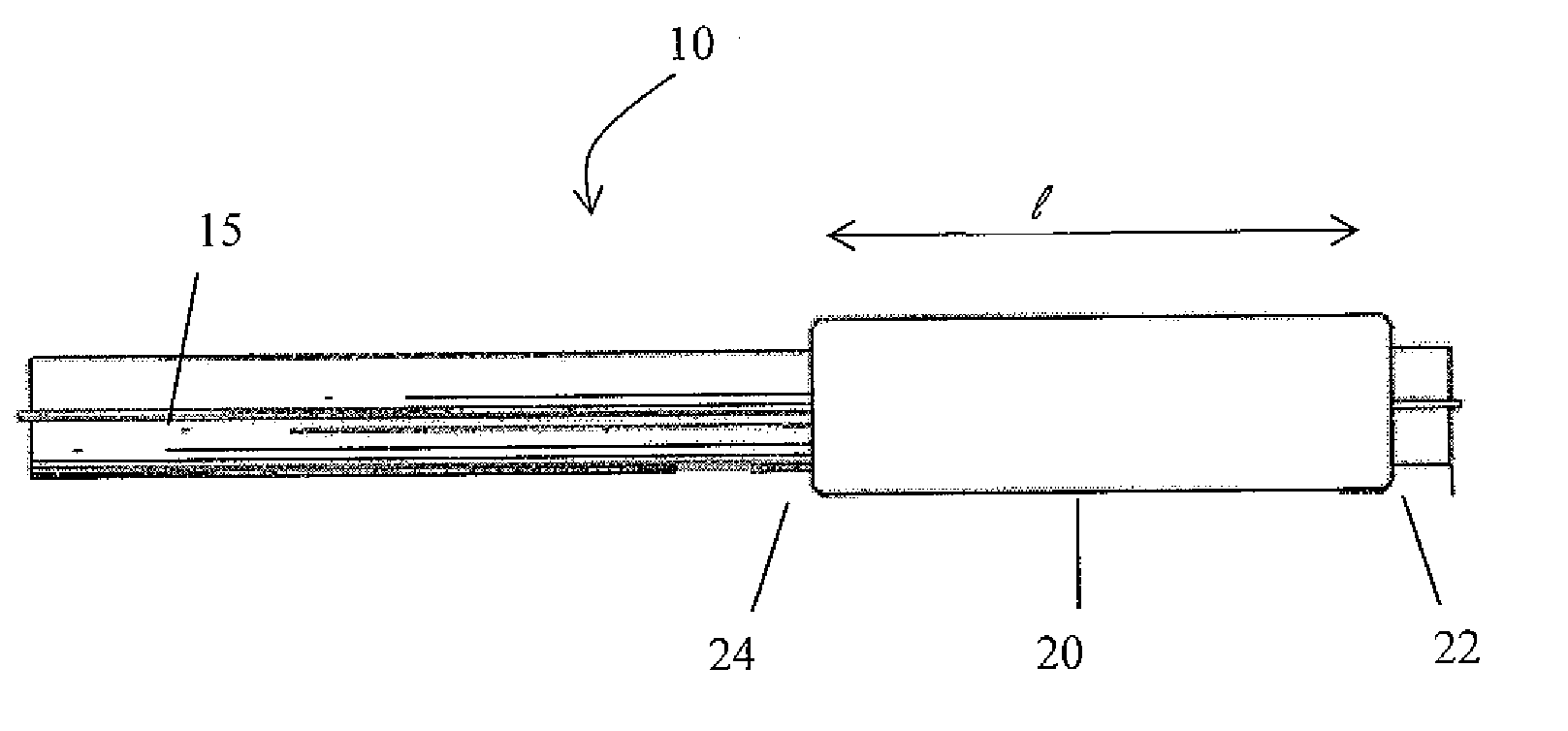
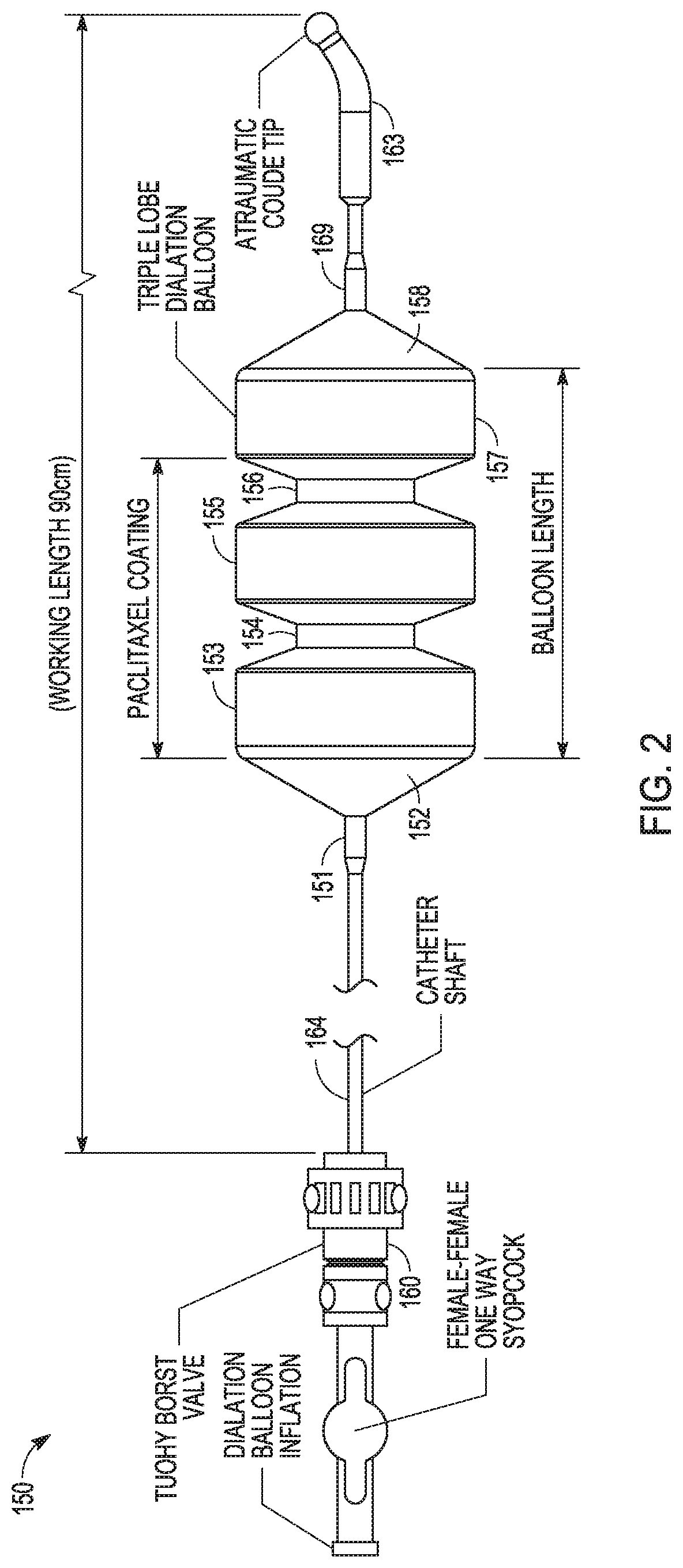
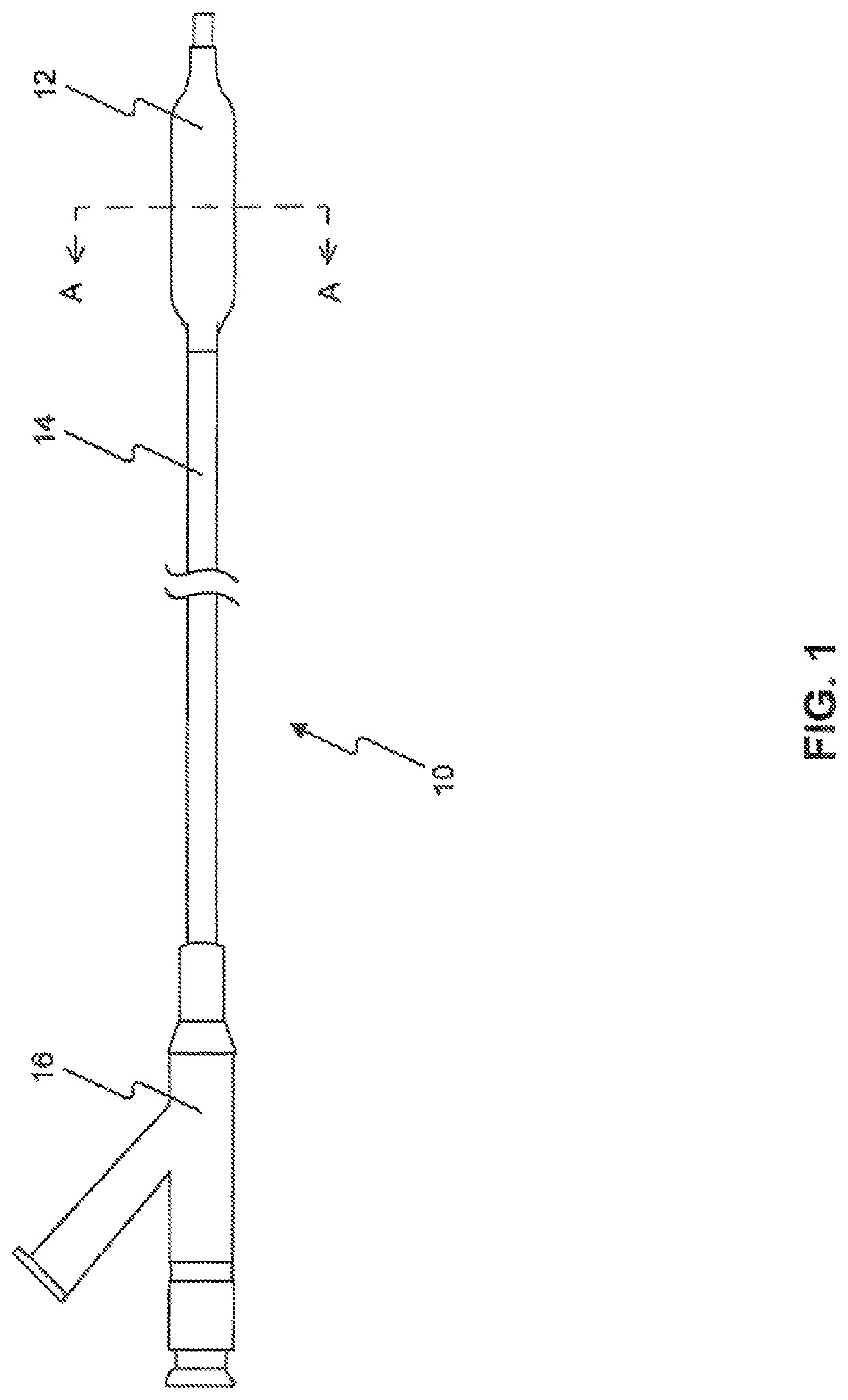
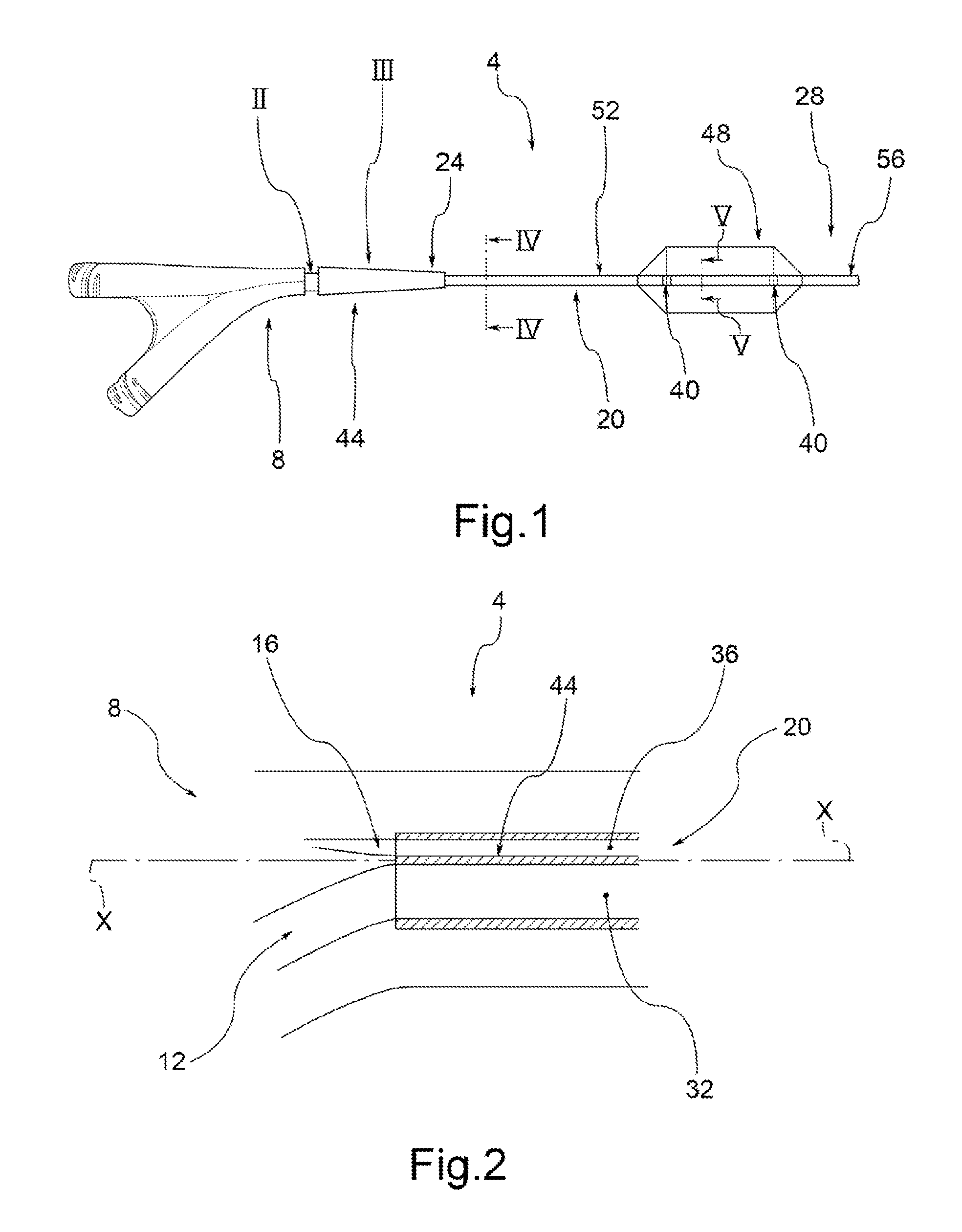
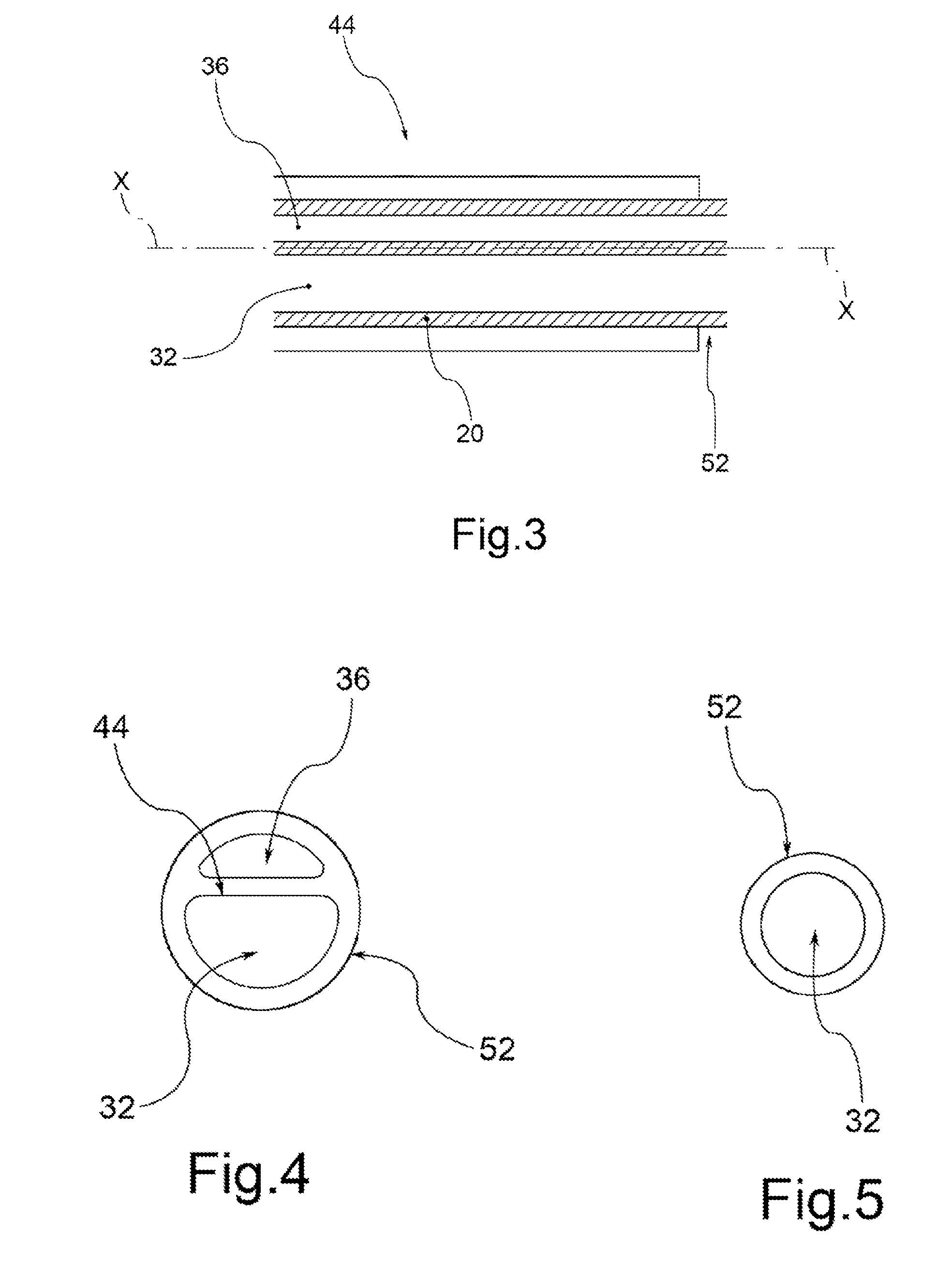
Drug Coated Balloon Catheter and Method of Manufacture Thereof
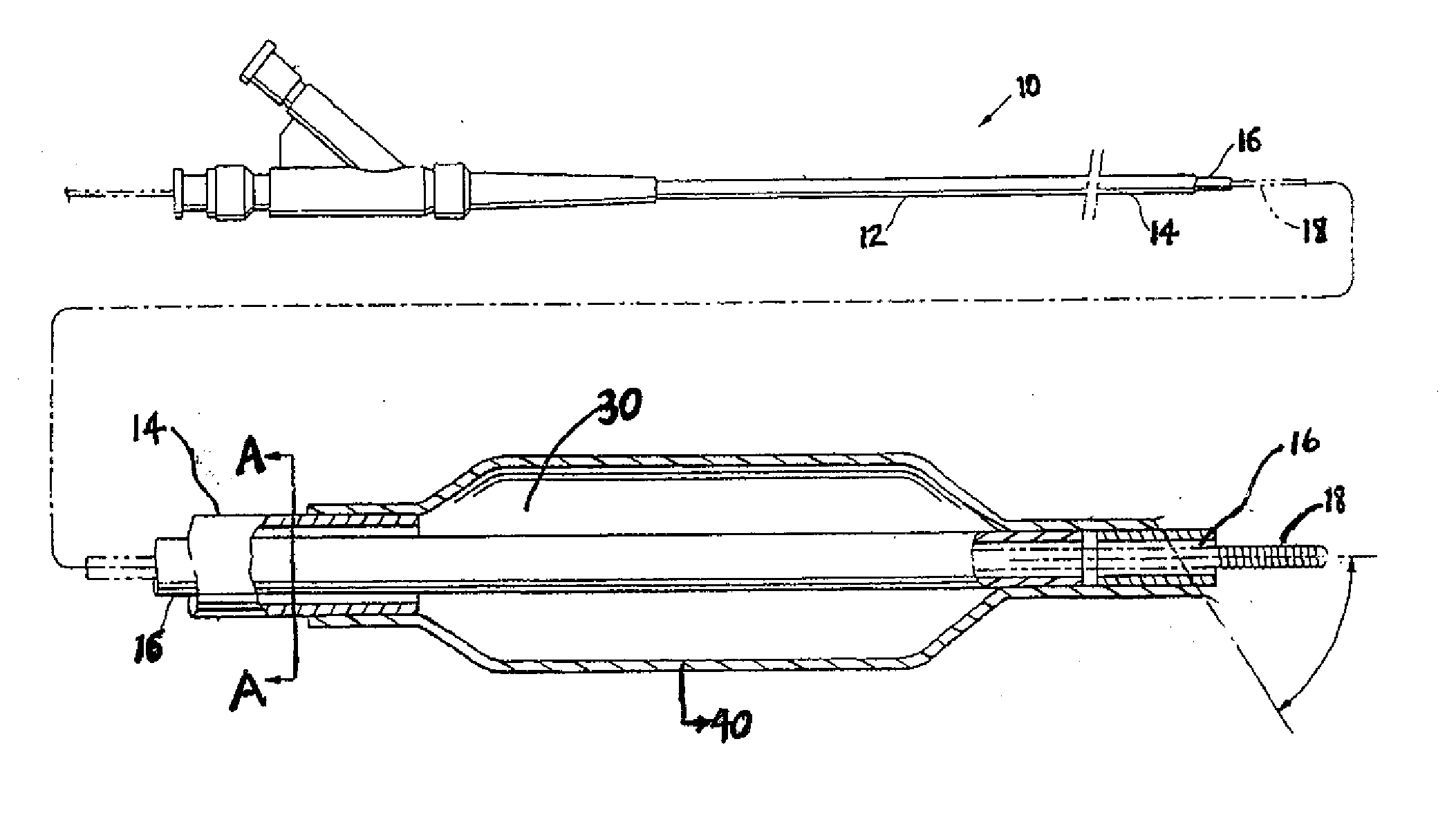
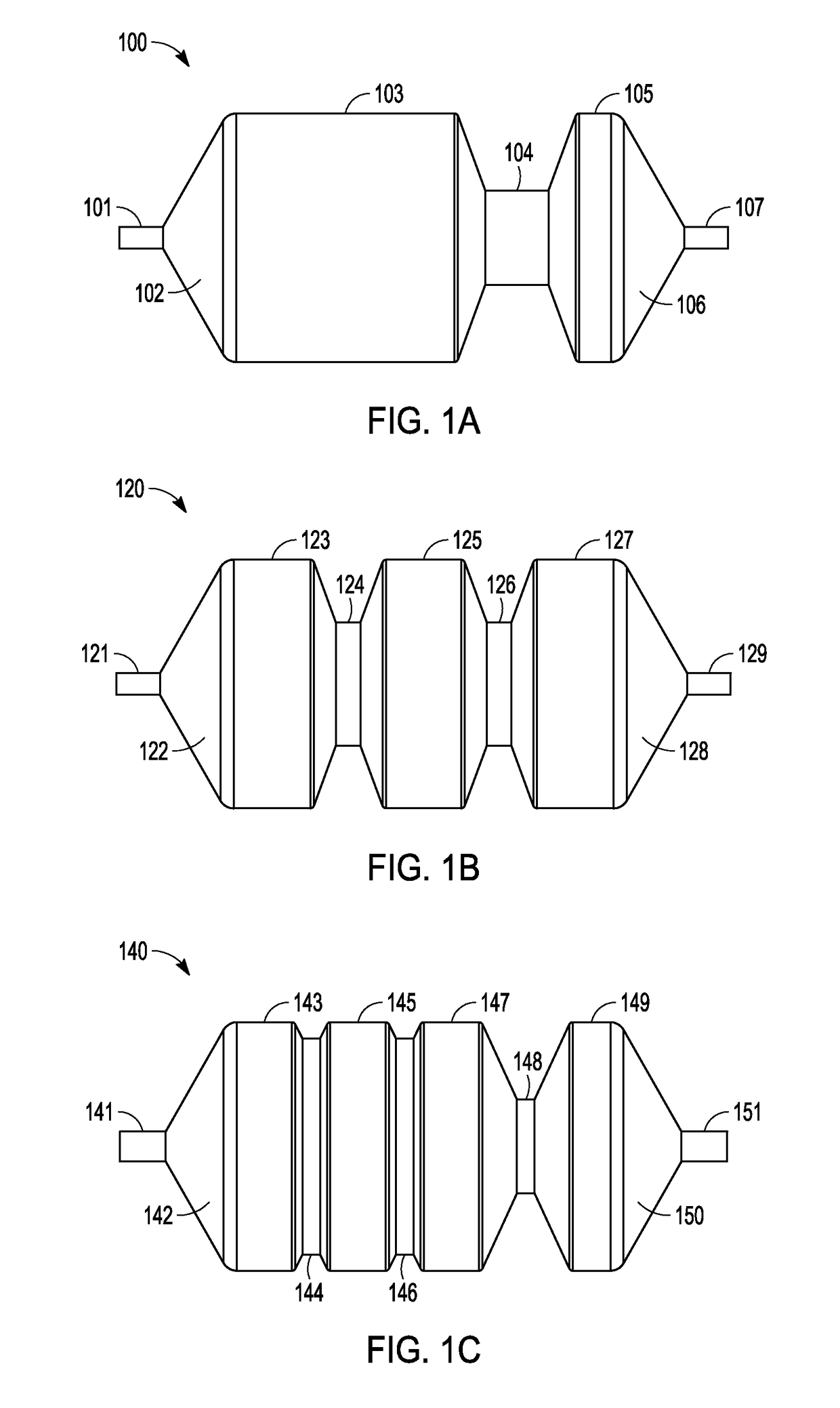
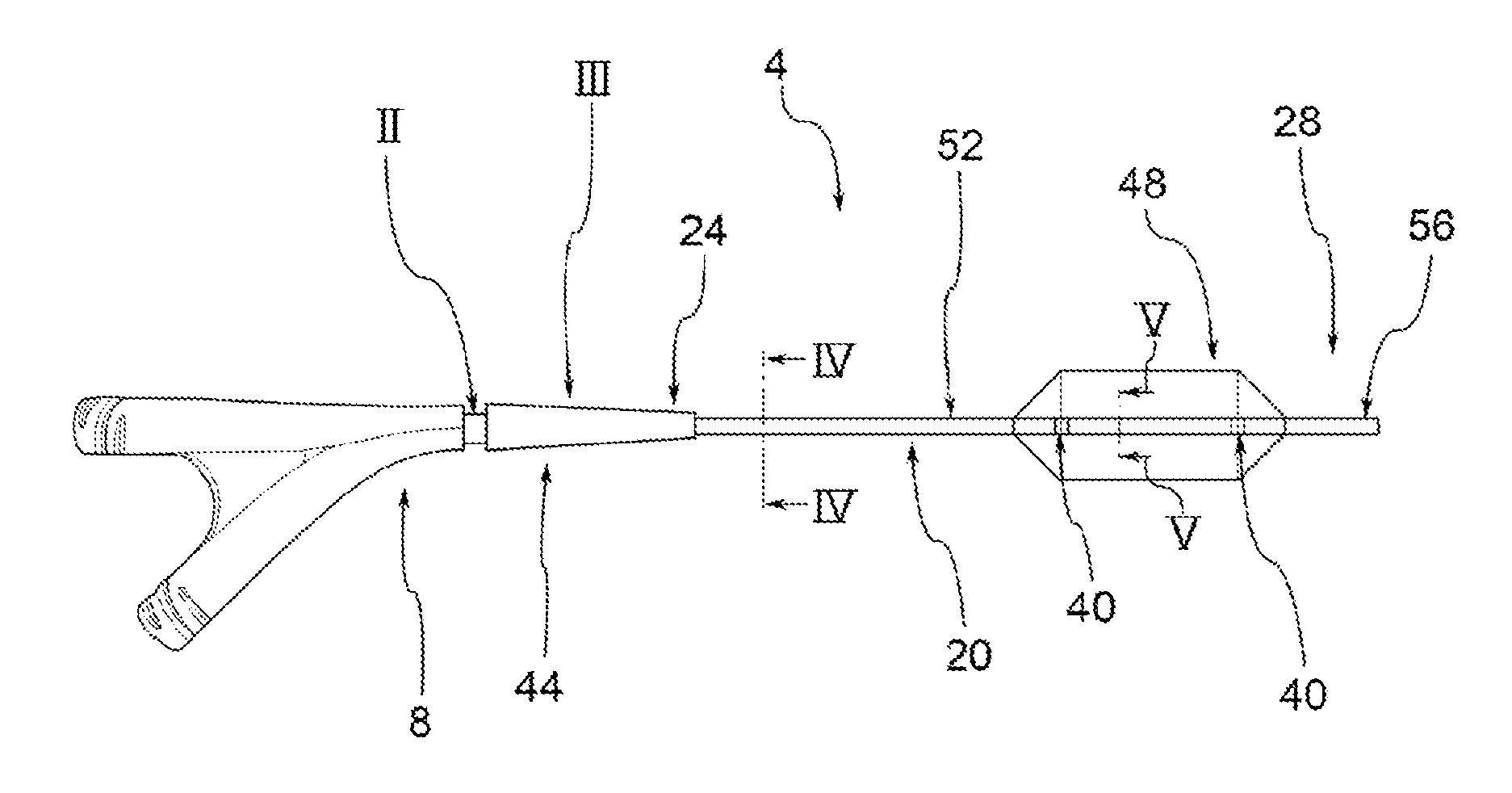
A drug coated balloon (DCB) catheter (4) has a connector (8), a shaft (20) extending from a proximal end (24) to a distal end (28) along an axial direction (X-X) and has a guidewire lumen (32) and an inflation lumen (36), the shaft being connected to the connector (8) on said proximal end (24), wherein the shaft (20) being provided with an inflatable balloon (48), fluidically connected with said inflation lumen (36) in order to be selectively inflated and / or deflated. Advantageously, the balloon (48) is coated with a drug to be delivered on a target lesion, an external wall (52) of the shaft (20), opposite to said lumens (32,36), is covered with a lubricant, and said guidewire lumen (32) is internally covered with a lubricant.
Owner:PINE MEDICAL
Hydrophobic therapueutic agent and solid emulsifier coating for drug coated balloon
The disclosed subject matter is directed to a coated medical device such as a balloon or stent and methods of manufacturing the device, where the device has a working length disposed between a distal end and a proximal end thereof; and a coating applied to at least a length of the body. The coating includes a hydrophobic therapeutic agent having a water solubility less than about 15.0 [mu]g / ml and an emulsifier that is a solid at ambient temperature.
Owner:ABBOTT CARDIOVASCULAR
Drug coated balloon catheter
The present invention relates to balloon catheters for treating a luminal system of a patient. Specifically, the invention relates to catheters having a flexible membrane positioned at a distal portion of the catheter, the flexible membrane retained in a substantially unexposed conformation prior to deployment. Preferably the flexible membrane is capable of delivering a therapeutic agent to a localized environment when deployed to an exposed conformation.
Owner:ABBOTT CARDIOVASCULAR
Drug coated balloon hemostatic valve insertion/balloon sheath
Introducer sheath for use in inserting a balloon catheter into a patient's vasculature includes a tubular member having an inner diameter, an outer diameter, a proximal end, a distal end, and a length therebetween, the inner diameter being sized to receive a catheter shaft having an attached expandable member in a deflated condition. The inner diameter can be varied from a first inner diameter at the proximal end and a second inner diameter at the distal end, the first inner diameter being greater than the second inner diameter. A method of inserting a balloon catheter into the vasculature of a patient is also disclosed. Additionally, a balloon catheter kit including a catheter and an introducer sheath is disclosed.
Owner:ABBOTT CARDIOVASCULAR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com