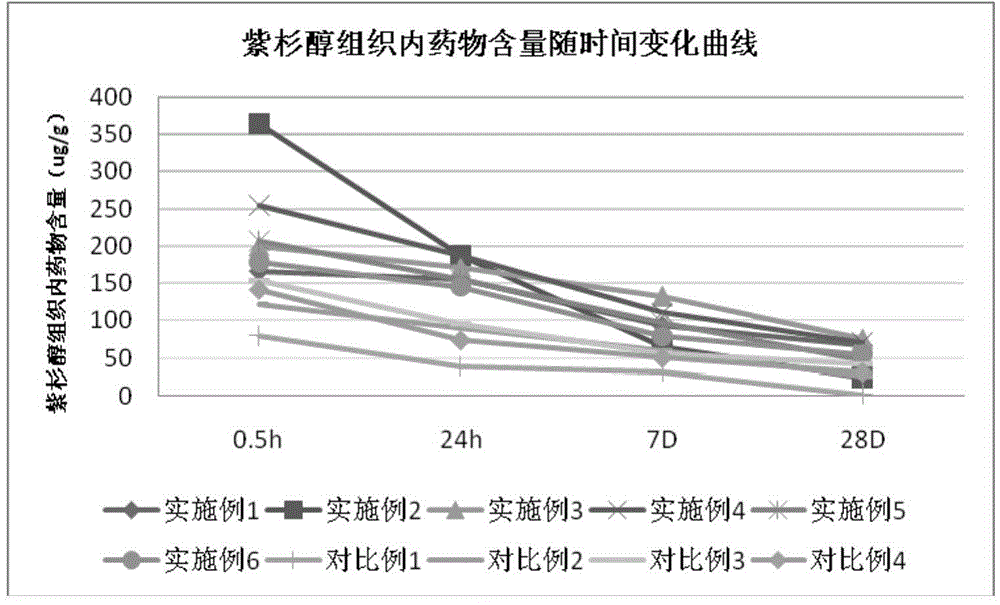
Drug-coated balloon catheter
A technology of drug-coated and balloon catheters, applied in catheters, coatings, medical science, etc., can solve the problems of restenosis of blood vessels, high restenosis rate of bare balloons, failure to show effectiveness or safety, and achieve Prevent large-area agglomeration, reduce the probability of agglomeration, and reduce the effect of coating safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 250mg paclitaxel, 5.0mg citrate, 0.1mg aminoalcohol, 10ml ethanol, 4ml purified water were mixed to prepare a coating solution, wherein the mass ratio of the active drug to the carrier was 49; the PTA balloon catheter (diameter 4mm, long 40mm) After the flaps were folded in three in a class 10,000 clean environment, the coating solution was drip-coated with a precision syringe (accurate to 2 μl) onto the surface of the polyester balloon behind the flaps in a class 100 clean environment, and then the balloon was dried , repeated dripping until the drug concentration on the surface of the balloon reached 20 μg / mm 2 , dried for 24 hours, packaged, and ethylene oxide sterilized.
Embodiment 2
[0039]11mg rapamycin, 17mg lactate, 5mg mannitol, 7ml ethanol, 3ml purified water were mixed to prepare a solution, wherein the mass ratio of the active drug to the carrier was 0.5; the PTA balloon catheter (diameter 4mm, long 40mm) was placed on After the flaps were folded in three in a class 10,000 clean environment, the coating solution was drip-coated on the surface of the polyester balloon with a precision syringe (accurate to 2 μl) in a class 100 clean environment, and then the balloon was dried and repeated. Drop coating until the drug concentration on the surface of the balloon reaches 3 μg / mm 2 , dried for 24 hours, packaged, and ethylene oxide sterilized.
Embodiment 3
[0041] 120mg paclitaxel, 36mg sodium benzoate, 12mg polyethylene glycol 2000, 10ml ethanol, 4ml purified water were mixed to prepare coating solution, wherein the mass ratio of active drug and carrier was 2.5; the PTA balloon catheter (diameter 4mm, long 40mm) After the wings were folded in three in a class 10,000 clean environment, the coating solution was sprayed onto the surface of the polyester balloon after the flaps were folded in a class 100 clean environment, so that the drug concentration on the surface of the balloon reached 3 μg / mm 2 , dried, packaged, and ethylene oxide sterilized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com