An acid-responsive nanometer micelle for drug loading, a preparation method and an application thereof
A nano-micelle, acid technology, applied in the field of acid-responsive nano-micelles and their preparation, can solve the problems of unsatisfactory effect, killing tumor cells, slow drug release, etc., to enhance anti-tumor effect, improve effect, increase tissue permeability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] In some embodiments, a method for preparing ultra-sensitive slightly acidic environment-responsive nanomicelles is provided, comprising the following steps:
[0042] S1. Synthesis of aminated PEG-NH with PEG-OH as raw material 2 ;
[0043] S2. Aminated PEG-NH 2 As the initiator, in freshly distilled CHCl 3 Initiate ring-opening polymerization of BLA-NCA in anhydrous DMF mixed solvent to obtain PEG-PBLA;
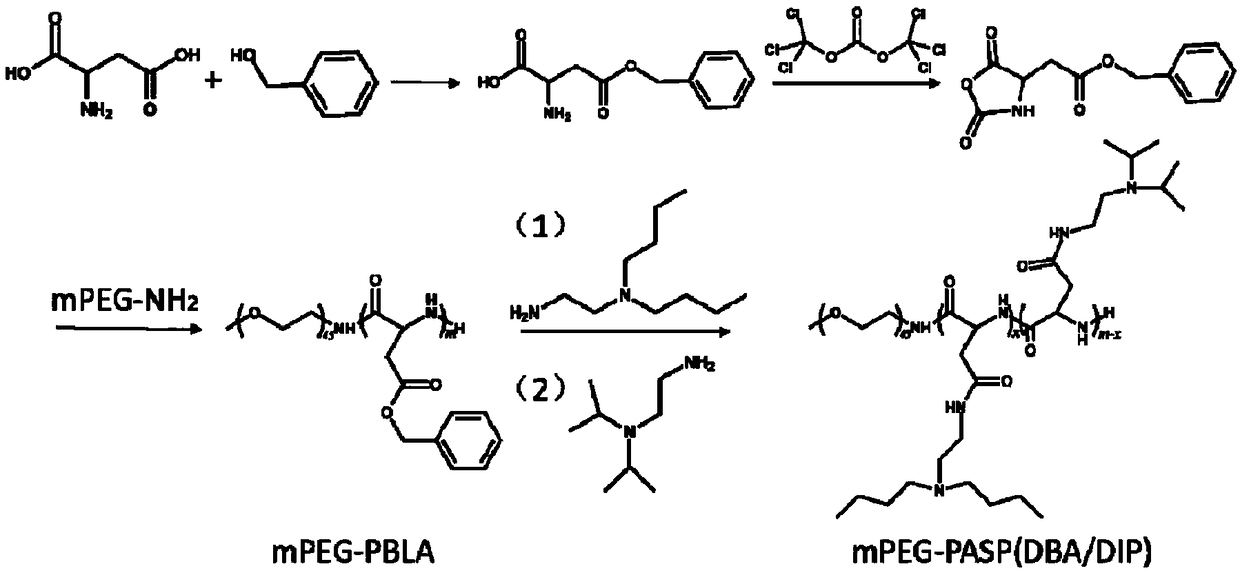
[0044] S3. Using PEG-PBLA as raw material, add DIP and DBA (1:0; 3:1; 1:1; 1:3; 0:1) in anhydrous DMSO according to five different molar ratios for ammonolysis to obtain PEG-PAsp (DIP / DBA).
[0045] In some embodiments, S1 uses PEG-OH as raw material to synthesize PEG-NH 2 The specific steps are: the PEG-OH with freshly distilled CHCl 3 After dissolving, add DMAP, triethylamine and p-toluenesulfonyl chloride at 0°C, stir at room temperature for 12 hours, precipitate in a large amount of ether, and filter and dry. Add the solid to a large amount of ammonia water ...
Embodiment 1
[0054] An embodiment of the acid-responsive nanomicelle used for drug loading in the present invention is a two-block polymer formed by the self-assembly of a hydrophilic block and a hydrophobic block; wherein, the hydrophilic block is PEG, and the hydrophobic block is PEG. The neutral block is PAsp(DIP / DBA); the preferred molecular weight of PEG is 2kDa, and the preferred molecular weight of PAsp(DIP / DBA) is 10kDa.
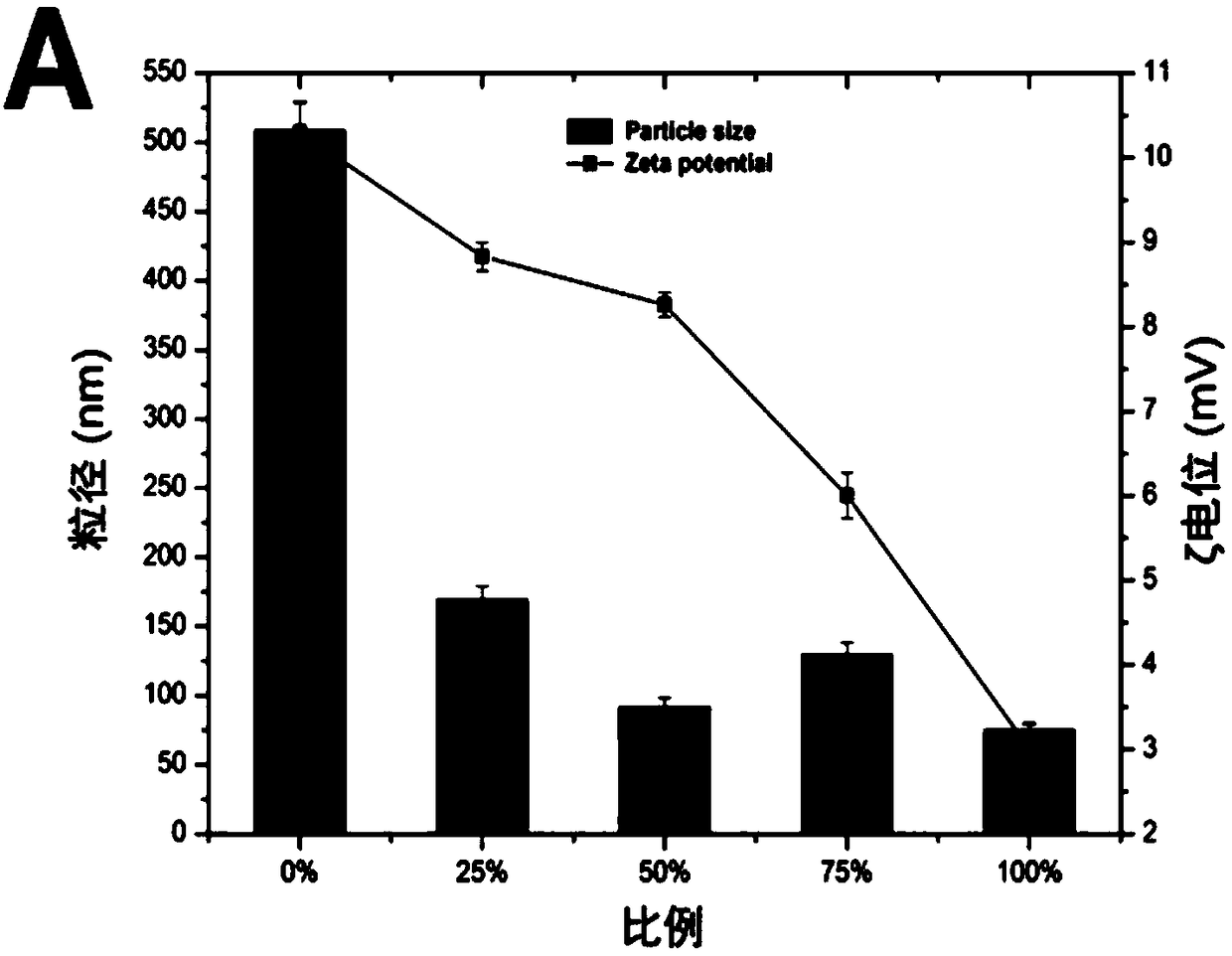
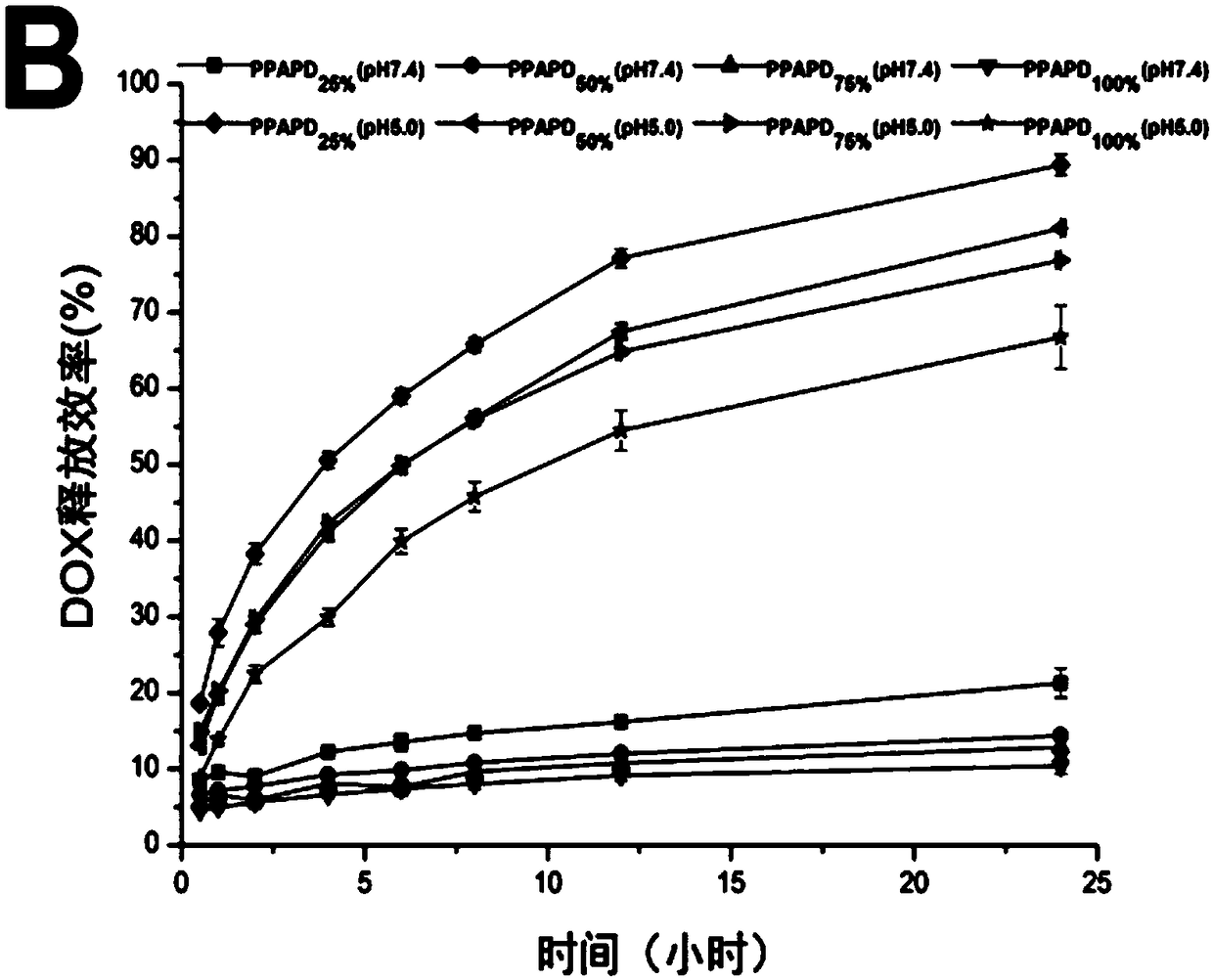
[0055] An embodiment of the anti-tumor nano-medicine in the present invention is composed of the above-mentioned nano-micelle loaded with doxorubicin and / or SPIO; wherein, the particle size of the anti-tumor nano-medicine is 130.5±8.0nm.
[0056] An embodiment of the preparation method of the anti-tumor nano-medicine in the present invention includes the following steps: using an emulsification method to ultrasonically induce the assembly of the above-mentioned nano-micelle, doxorubicin and SPIO to prepare an anti-tumor nano-drug visualized by magnetic resonance. ...
Embodiment 2
[0057] The synthesis of embodiment 2 block polymers (i.e. nano micelles)
[0058] A kind of embodiment of the preparation method of nano-micelle of the present invention, the synthetic route of block polymer (nano-micelle) is as follows figure 1 As shown, it specifically includes the following steps:
[0059] First, sulfonated PEG was synthesized from PEG-OH. Specifically, 7.0 g of PEG-OH was first mixed with 50 mL of anhydrous CHCl 3 Dissolve, then add 43mg DMAP, 0.73mL triethylamine and 1.0g p-toluenesulfonyl chloride at 0°C, stir and react at room temperature for 12 hours, then precipitate the reaction solution in a large amount of anhydrous ether (more than 8 times the volume of the reaction solution) ), filtered to obtain a solid, and obtained a solid sulfonated PEG after vacuum drying;
[0060] Then, the solid was added to a large amount of ammonia water (23%-28%), sealed, stirred for at least 5 days, concentrated, and washed with CHCl 3 After extraction, add dilute ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com