Immediate release formulations of 1-aminocyclohexane compounds, memantine and neramexane
a technology of neramexane and memantine, which is applied in the direction of antiparasitic agents, biocides, drug compositions, etc., can solve the problems of not specifically revealing compositions containing memantine or neramexane, unpleasant taste of casein, etc., and achieve dose-proportional bioavailability and advantageous effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Memantine HCl Immediate Release Tablets
[0213] The present example describes the process of developing memantine hydrochloride immediate release tablets in 2.5, 5, 10, 15, 20, 40, 60, and 80 mg dosages.
Materials and Methods
[0214] The following tables provide the makeup of immediate release tablets including the active components, coating agent, and other excipients for the specified dosage forms with specific target release time periods. Tables 1 and 2 provide the makeup of tablets with lactose and contain the same data expressed respectively in absolute (mg) or relative (% w / w) terms.
TABLE 12.5 mg to 80 mg Dose Proportional Formulations (with lactose / MCC)Component oringredient (mg)Content (mg)Memantine HCl2.55101520406080Microcrystalline13.0326.0552.1078.15104.20208.40312.60416.8CelluloseLactose Monohydrate43.6987.38174.75262.13349.50699.001048.501398.0Colloidal0.320.631.251.882.505.007.5010.0SiliconeDioxideTalc2.795.5711.1516.7222.3044.6066.9089.2Magnesium Stear...
example 2
Pharmacokinetic Study of Memantine
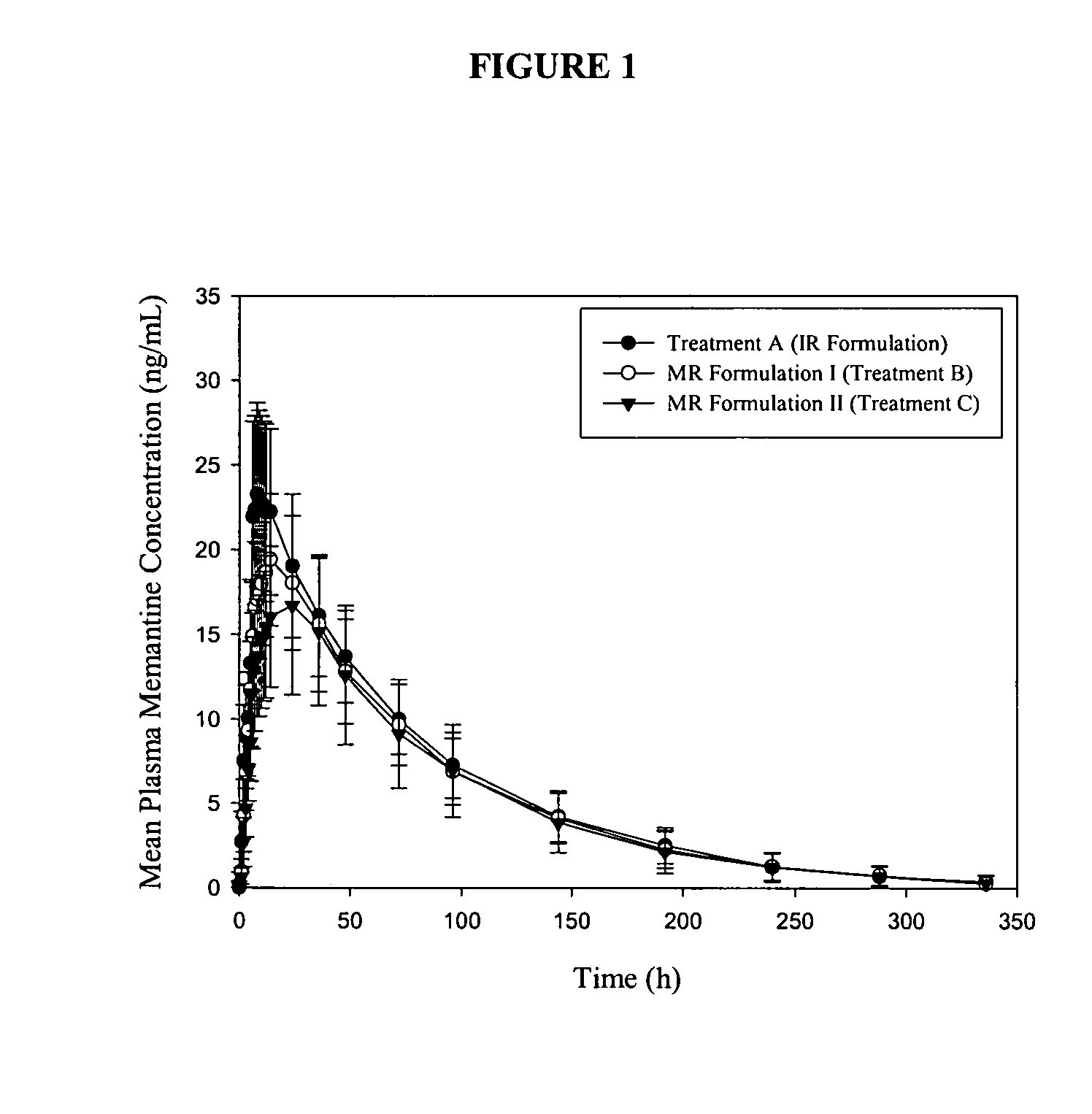
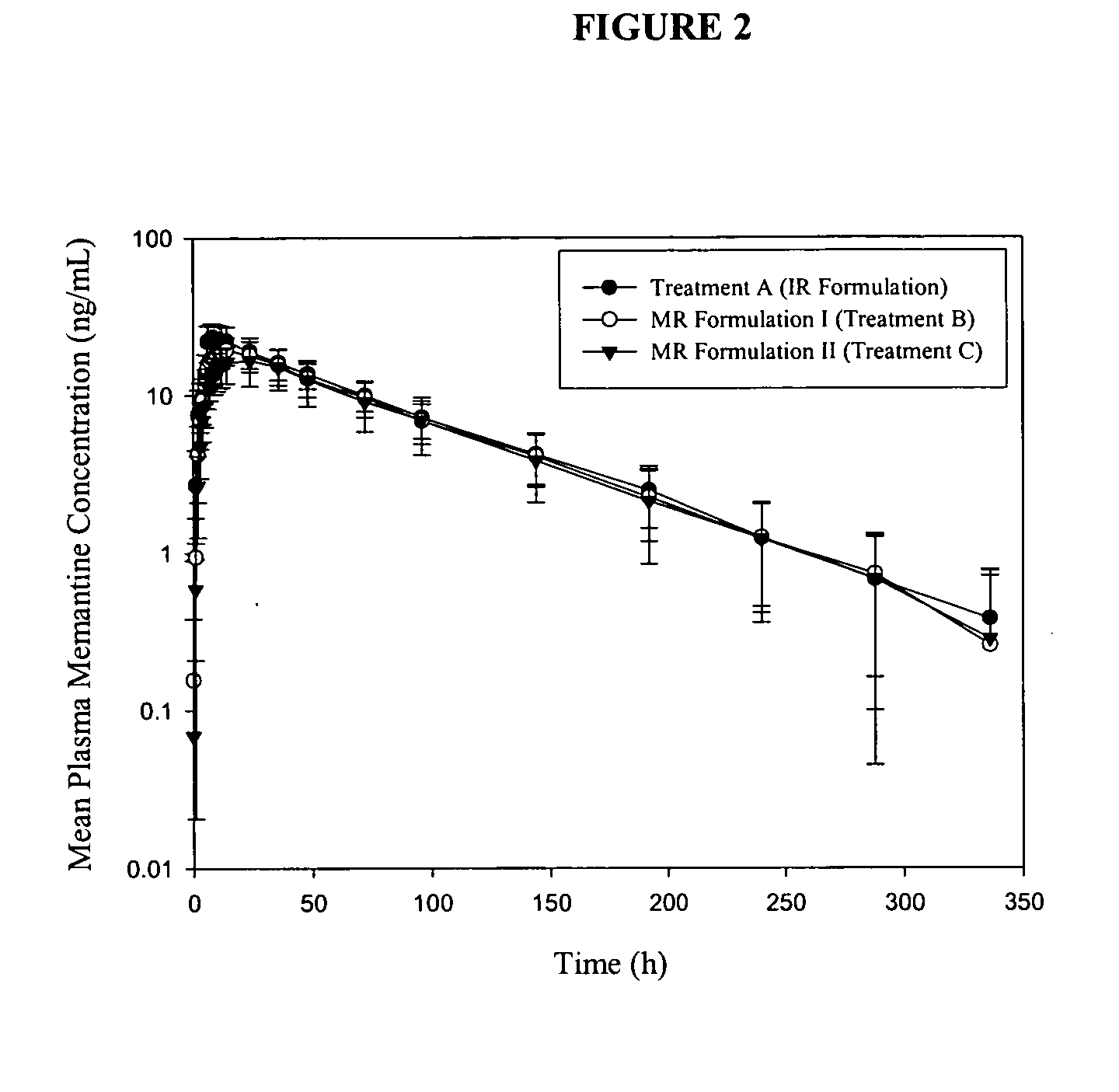
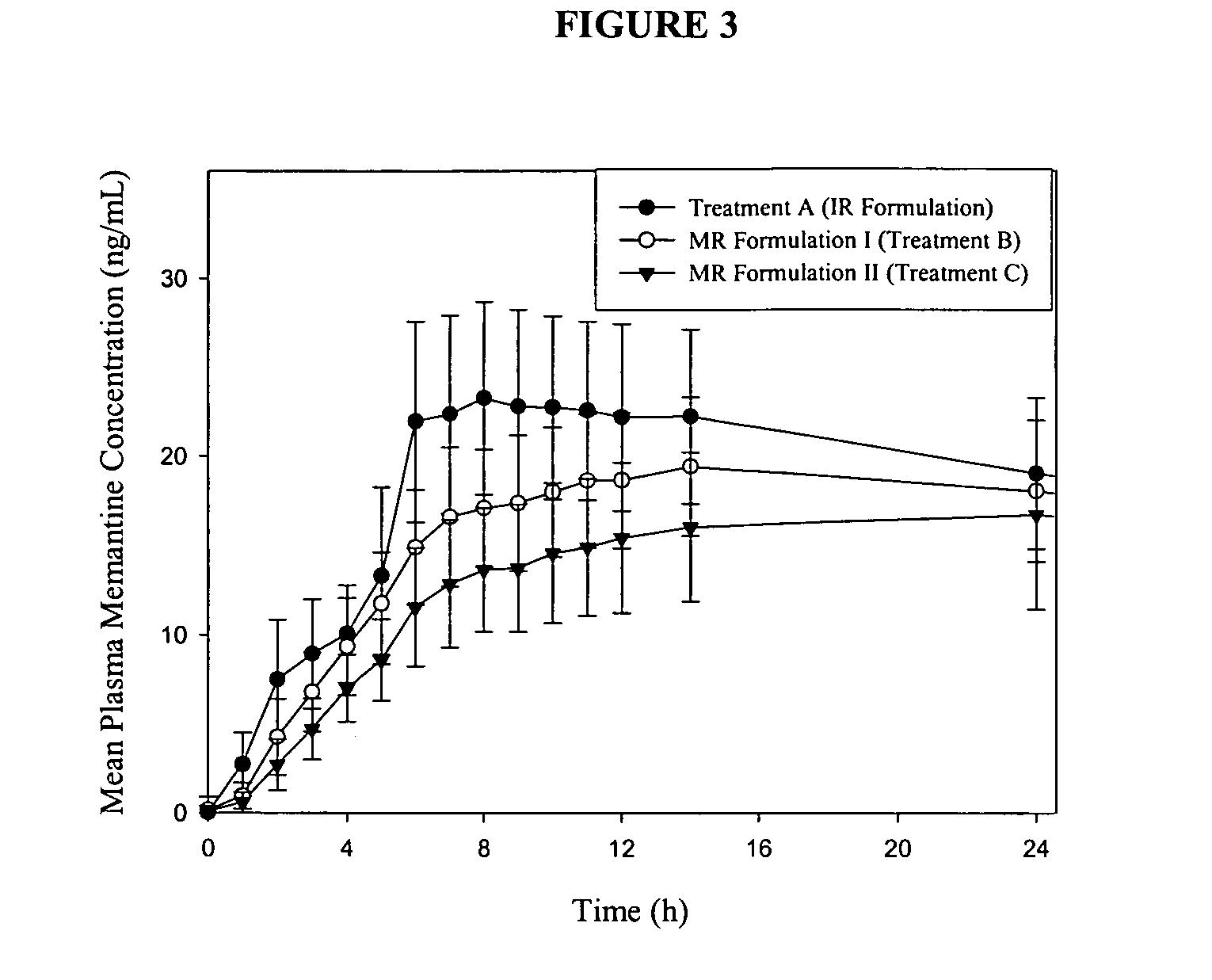
[0237] The present example presents the bioavailability of immediate release memantine tablets as compared to modified release memantine tablets.
Materials and Methods
[0238] The study design in the present example was a 57-day single-center, open-label study in 24 young healthy subjects, ages ranging from 18 to 35 years old. Subjects underwent a screening evaluation consisting of a complete medical history, complete physical examination with vital signs, 12-lead ECG, clinical laboratory evaluations, consisting of a CBC (including differential), clinical chemistry, urinalysis, RPR / VDRL, Anti HIV 1 and 2 tests, drugs of abuse screen (including alcohol and nicotine), Anti-HCV and HbsAg. Female subjects had a β-hCG serum pregnancy test performed at screening and a urine pregnancy test on Day -1.
[0239] Inclusion criteria included informed consent, normal physical examination, healthy adults between 18 and 35 years of age, non-smokers, within 15% of id...
example 3
Preparation of Memantine HCl 30-minute Immediate Release Tablets
[0265] The present example demonstrates the makeup of 30-minute immediate release memantine tablets, with and without lactose monohydrate.
[0266] The methods of making the tablets are the same as those disclosed in Example 1. Specifically, the tablets are made of the following active components, coating agent, and other excipients as presented below in Tables 9 and 10. Tables 9 and 10, summarizing the tablets with lactose monohydrate, contain the same data expressed respectively in absolute (mg) or relative (% w / w) terms.
TABLE 930 min release tablets with lactose monohydrate / MCC(weights in mg / tablet)PreferredComponent / Ingredient (mg)RangesExact Composition (mg)Memantine HCl5.080.05.010.015.020.0Microcrystalline Cellulose23.4458.526.152.178.2104.2Lactose Monohydrate78.61537.987.4174.8262.1349.5Colloidal Silicone Dioxide0.611.10.61.31.92.5Talc5.098.05.611.216.722.3Magnesium Stearate0.36.50.40.81.11.5Hydroxypropyl3.466....
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com