Compound nateglinide valsatan medicinal composition
A technology of nateglinide and composition, applied in the field of double-layer tablet and preparation thereof, can solve the problems of inability to carry out satisfactory dissolution, poor disintegration, inability to exert quick effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
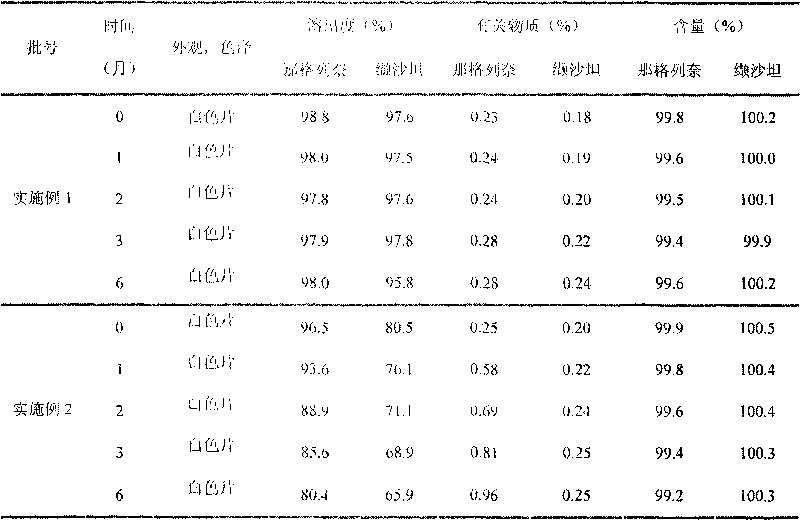
Embodiment 1
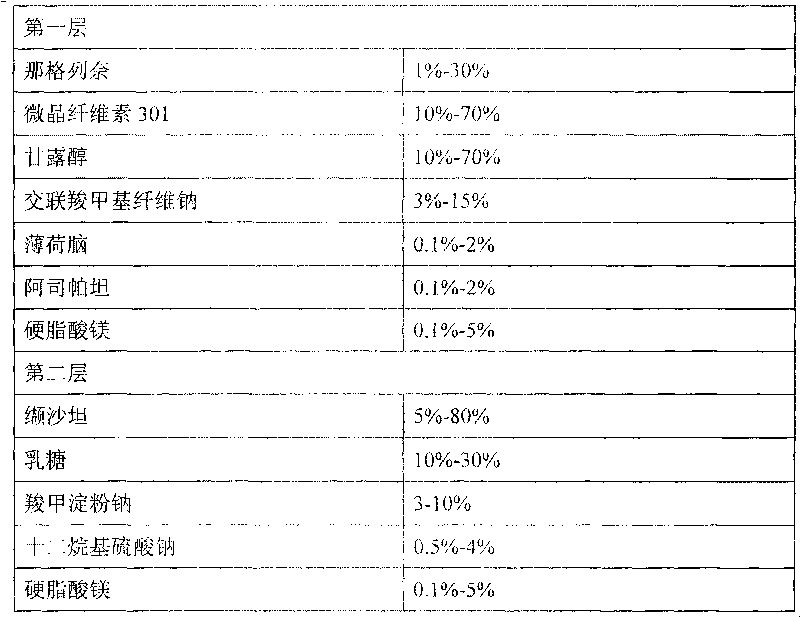
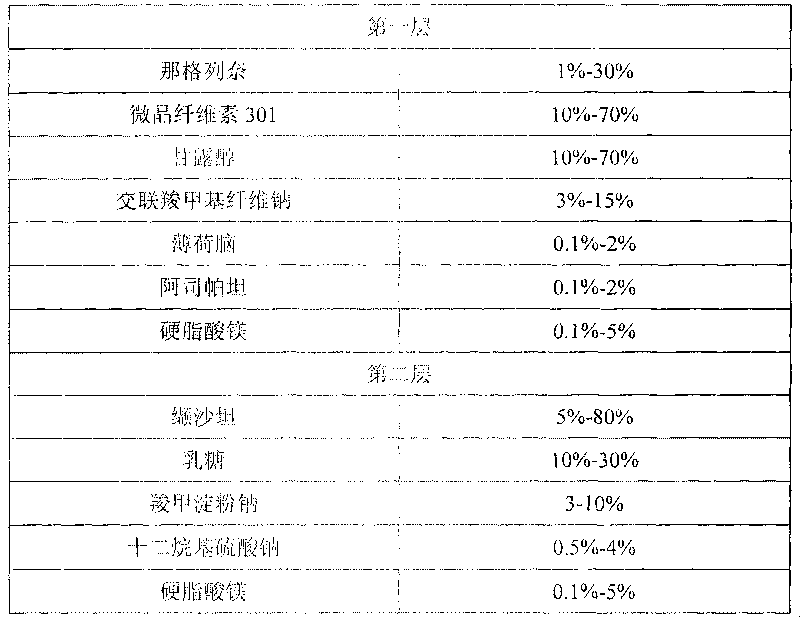
[0052] prescription composition
[0053]
[0054] Preparation Process
[0055] 1) Preparation of the first layer: Weigh nateglinide, microcrystalline cellulose 301, mannitol, menthol, aspartame, croscarmellose sodium, and mix them uniformly with 95% ethanol solution to make soft material, granulate with 12-20 mesh screen, dry at 30-70°C, granulate with 12-20 mesh screen, add magnesium stearate, mix evenly, and press the first layer.
[0056] 2) To prepare the second layer of granules, weigh valsartan, lactose, sodium carboxymethyl starch, and sodium lauryl sulfate, and mix them uniformly by adding in equal amounts, then use 50% ethanol solution to make a soft material, 12- Granulate with a 20-mesh screen, dry at 30-70°C, granulate with a 12-20-mesh screen, add magnesium stearate, mix evenly, and press the second layer.
[0057] 3) Tablet compression: After adjusting the weights of the two layers, press them into double-layer tablets with a multifunctional tablet press.
Embodiment 2
[0058] Embodiment 2 (prior art)
[0059] prescription composition
[0060] Naglinide
30mg / tablet
Microcrystalline Cellulose 301
40mg / tablet
40mg / tablet
8mg / tablet
0.5mg / tablet
[0061] Naglinide
30mg / tablet
0.5mg / tablet
80mg / tablet
30mg / tablet
8mg / tablet
0.5mg / tablet
2.5mg / tablet
[0062] Preparation Process
[0063] Weigh nateglinide, microcrystalline cellulose 301, mannitol, menthol, aspartame, croscarmellose sodium, valsartan, lactose, carboxymethyl starch sodium, lauryl sulfate Sodium, mix evenly by adding equal amount, make soft material with 75% ethanol solution, granulate with 12-20 mesh screen, dry at 30-70°C, granulate with 12-20 mesh screen, add magnesium stearate, Mix evenly, press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com