Intraorally disintegrating valdecoxib compositions
A technology of valdecoxib and its composition, which is applied in the field of treating cyclooxygenase-2-mediated diseases, and can solve problems such as low water solubility and delayed onset of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
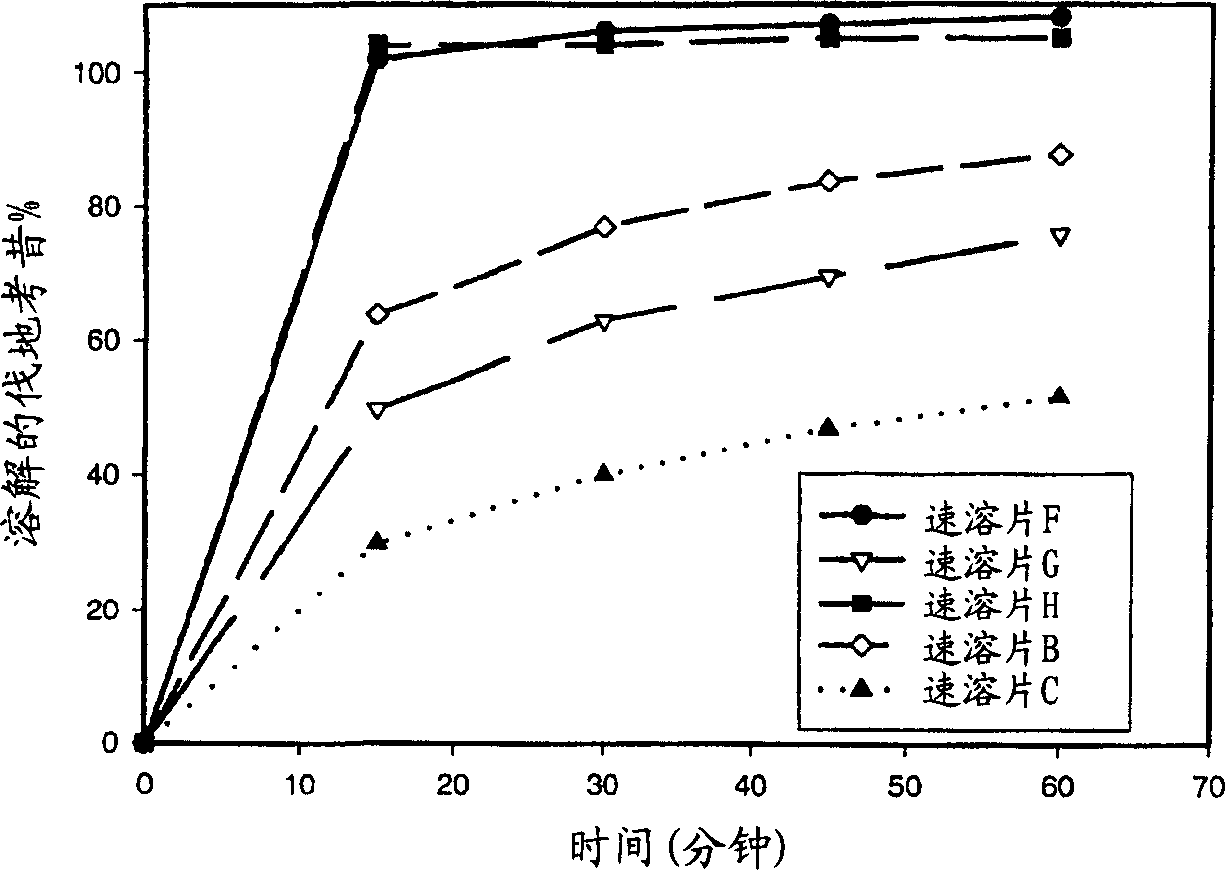
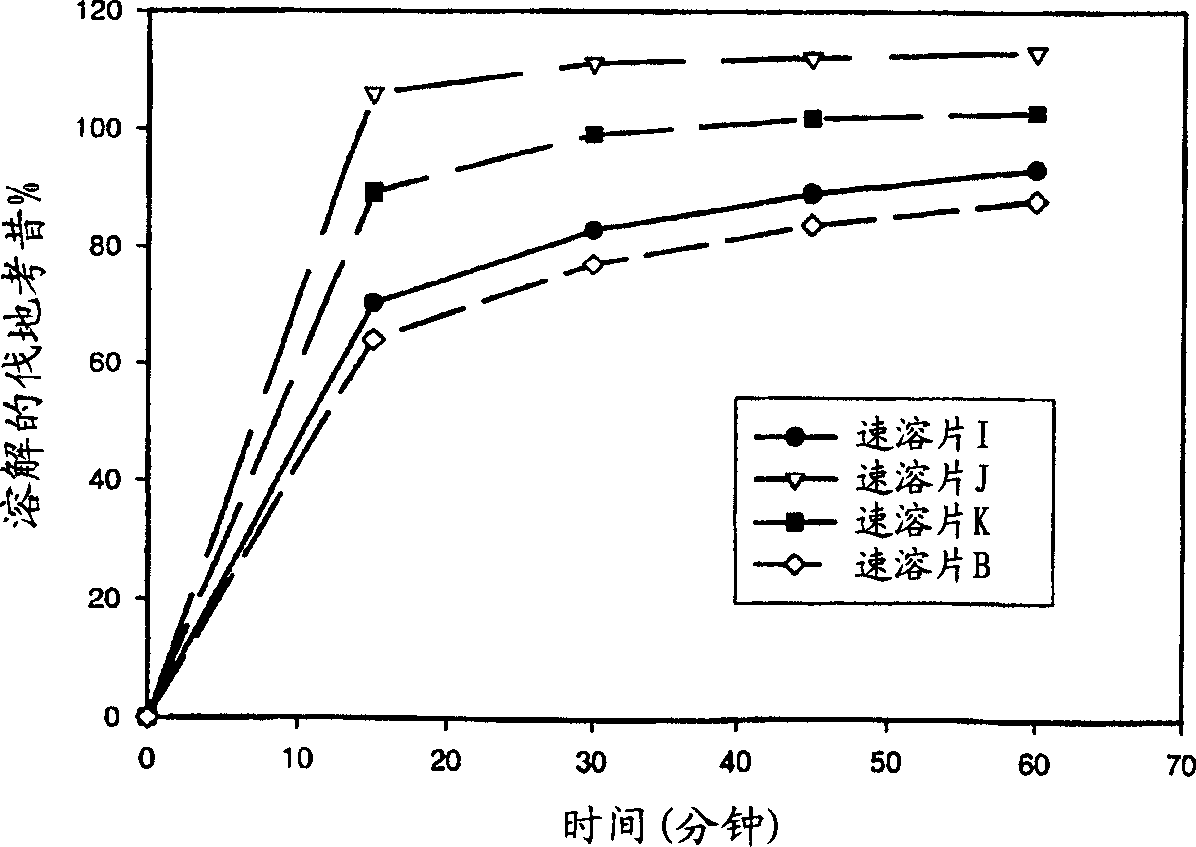
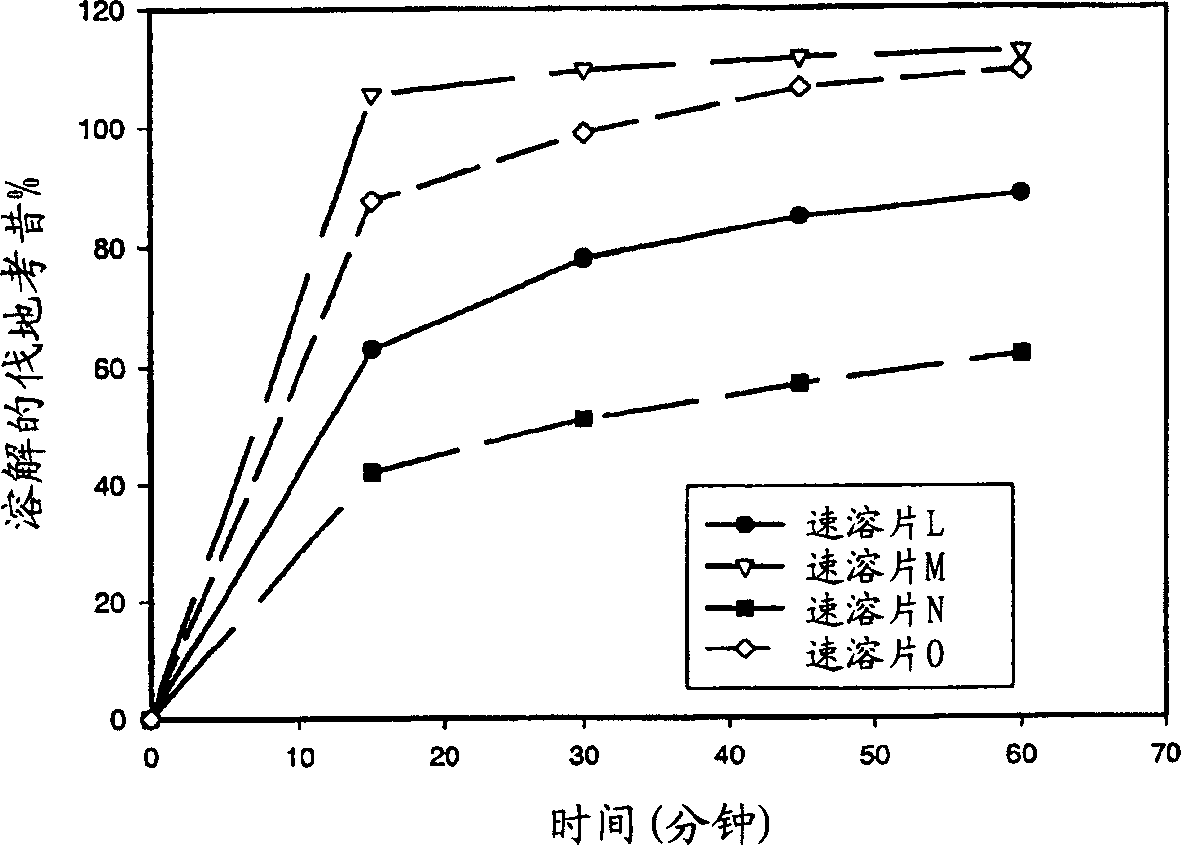
Image
Examples
Embodiment 1
[0125] Three kinds of valdecoxib composite granules (G1-G3) were prepared according to the following methods. Three batches of granulation fluid were prepared, as shown in Table 1, to prepare a dry powder blend comprising valdecoxib and at least one of Avicel PH101, PVP (K29-32) and sodium lauryl sulfate (SLS). The dry powder blend was wet granulated in a 2 liter Key Granulator.
[0126] Valdecoxib composite granules G1 were prepared using Eudragit(R) E PO, SLS and dibutyl sebacate and dispersed in 97.6 g of water; this dispersion was added to the dry powder blend over 4 minutes and mixed to form a mixture. An additional 30 g of water was then added to the mixture and the mixture was tray dried and hand passed through a 20 mesh screen to form valdecoxib composite granules.
[0127] Valdecoxib composite granules G2 were prepared using PVP as a dry binder. Water was added to the dry powder blend over 5 minutes. The granulation uniformity is poor, half of the raw material is s...
Embodiment 2
[0131] Prepare valdecoxib instant tablets (A batch, hereinafter also referred to as instant tablets A) according to the following method, and the components are shown in Table 2. Valdecoxib (457.75 g) and Avicel PH101 (226.92 g) were mixed together for 2 minutes in a Glatt granulator (main and cutter speeds set at 600 and 3000 rpm, respectively) to form a premix. Eudragit(R) E PO (49 g) and citric acid (16.33 g) were added to a vessel containing 250 g of water to form a solution. This solution was added to the premix at a substantially constant rate over 8.5 minutes (mixing continued) to form a wet mixture. After the solution addition was complete, the wet mixture was further mixed for 1 minute to form wet granules. The obtained wet granules are passed through a 18-mesh sieve, and dried in a 40° C. oven or a fluidized bed dryer to form a valdecoxib complex whose dissolution is retarded. Valdecoxib complex (98.31 g) was then blended with 483.69 g of placebo granules (composed...
Embodiment 3
[0134] Prepare valdecoxib instant tablets (batch B, hereinafter also referred to as instant tablets B) according to the following method, and the components are shown in Table 3. Valdecoxib (398.28 g) and Avicel PH101 (214.48 g) were mixed together for 2 minutes in a Glatt granulator (main and cutter speeds set at 600 and 3000 rpm, respectively) to form a premix. To a vessel containing 300 g of water was added Eudragit(R) E PO (112.15 g), sodium lauryl sulfate (7.88 g) and dibutyl sebacate (16.88 g) to form a dispersion. This dispersion was added to the premix at a substantially constant rate over 15 minutes (mixing continued) to form a wet mixture. After the addition of the dispersion was complete, the wet mixture was further mixed for 1 minute to form wet granules. The obtained wet granules are passed through a 18-mesh sieve, and dried in a 40° C. oven or a fluidized bed dryer to form a valdecoxib complex whose dissolution is retarded. Valdecoxib complex (112.99 g) was the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com