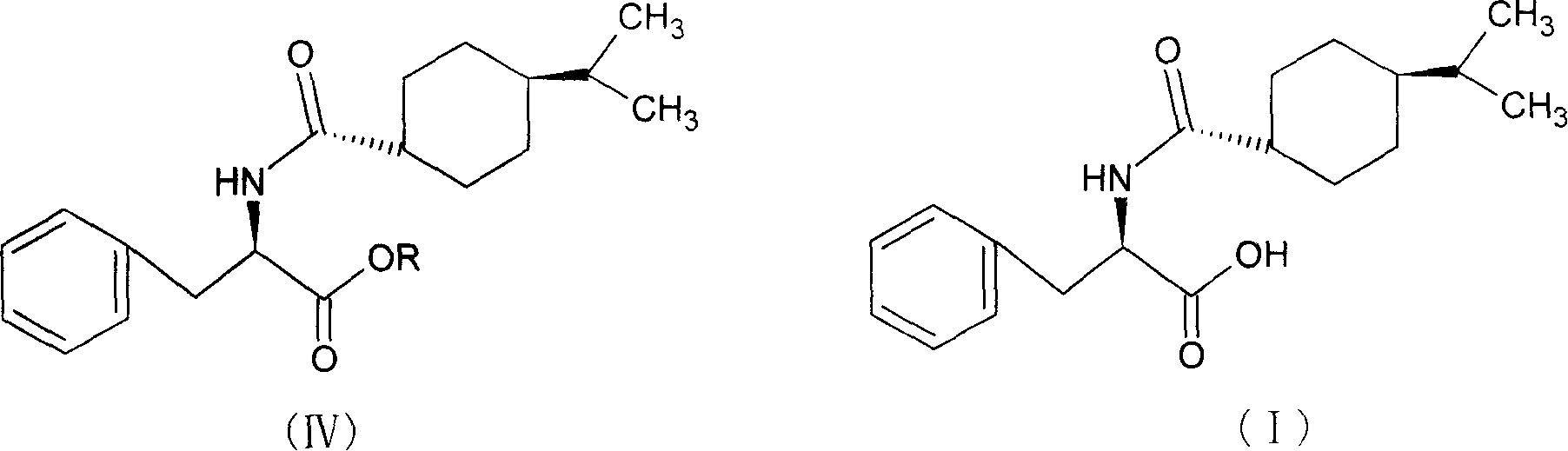
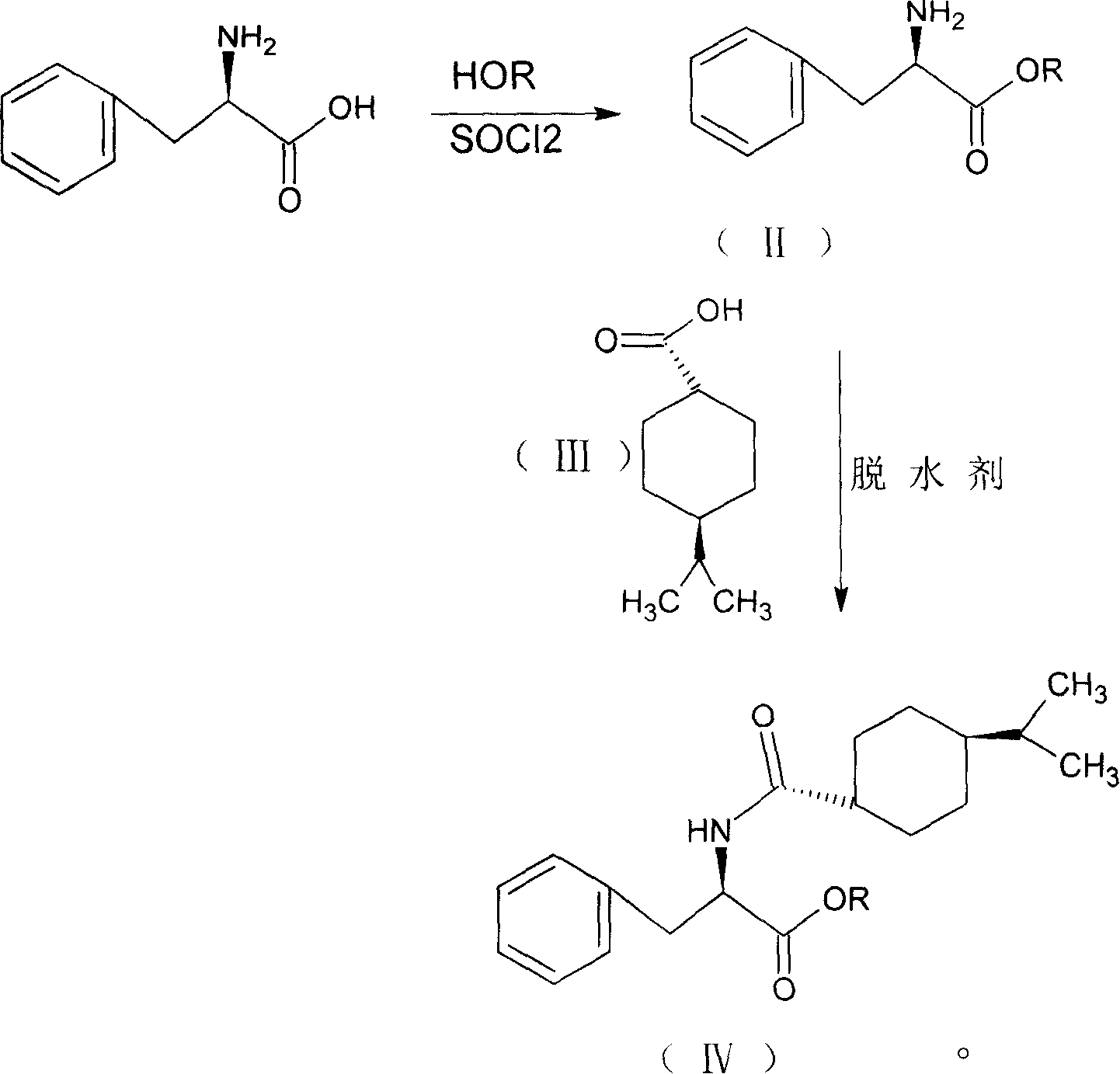
Synthesis of pharmaceutical
A technology for esters and compounds, which is applied to the field of one-pot synthesis of nateglinide, can solve the problems of complex processing, increased difficulty in industrialization, trouble, and the like, and achieves the effect of saving costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 100ml of methanol to the reaction flask, cool to 0-5°C, keep the temperature below 10°C, add 10ml of thionyl chloride dropwise, add 10g of D-phenylalanine, raise the temperature, keep at 40±2°C for 15 hours, After the reaction, distill off the methanol and precipitate the solid, add 25ml of dichloromethane, stir until the solid is completely dissolved, add 3.5g of 1-hydroxybenzotriazole, cool down to 0-5°C, add 9.5g of p-isopropylcyclohexanecarboxylic acid , keep the temperature below 5°C, add 25ml of dichloromethane dissolved with 12.5g N,N'-dicyclohexylcarbodiimide dropwise into the reaction solution in 1 hour, after the addition is completed, keep it at 10-15°C for 3 hours After the reaction is complete, use a funnel covered with celite to filter, use 25ml of 5% NaOH washing solution to wash the reaction solution twice, and then wash to neutrality. Evaporate dichloromethane, use 65ml of methanol for phase change, lower the temperature to 20-25°C, keep the tempera...
Embodiment 2
[0038] Add 100ml of ethanol to the reaction bottle, cool to 0-5°C, keep the temperature below 10°C, add 10ml of thionyl chloride dropwise, add 10g of D-phenylalanine, raise the temperature, keep at 40±2°C for 15 hours, After the reaction, the ethanol was evaporated and the solid was precipitated, and 25ml of chloroform was added, stirred until the solid was completely dissolved, then 3.5g of 1-hydroxybenzotriazole was added, the temperature was lowered to 0-5°C, 9.5g of p-isopropylcyclohexanecarboxylic acid was added, and kept When the temperature is lower than 5°C, 12.5g of N,N′-dicyclohexylcarbodiimide dissolved in 25ml of chloroform is added dropwise to the reaction solution in 1 hour. After the dropwise addition is completed, the reaction is kept at 10-15°C for 3 hours, and the reaction is completed. Use a funnel covered with celite to filter, wash the reaction solution twice with 25 ml of 5% NaOH washing solution, and then wash with water until neutral. Evaporate chlorofo...
Embodiment 3
[0041]Add 100ml of methanol to the reaction flask, cool to 0-5°C, keep the temperature below 10°C, add 10ml of thionyl chloride dropwise, add 10g of D-phenylalanine, raise the temperature, keep at 40±2°C for 15 hours, After the reaction, the ethanol was evaporated and the solid was precipitated. Add 25ml of tetrahydrofuran, stir until the solid was completely dissolved, add 3.5g of 1-hydroxybenzotriazole, lower the temperature to 0-5°C, add 9.5g of p-isopropylcyclohexanecarboxylic acid, and keep When the temperature is lower than 5°C, 25ml tetrahydrofuran dissolved with 12.5g N,N′-dicyclohexylcarbodiimide is added dropwise into the reaction solution in 1 hour. After the dropwise addition is completed, the reaction is kept at 10-15°C for 3 hours, and the reaction is completed. Evaporate tetrahydrofuran, use 65ml of methanol for phase change, lower the temperature to 20-25°C, keep the temperature below 30°C, add 23ml of 10% NaOH solution dropwise within 30 minutes, keep warm at 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com