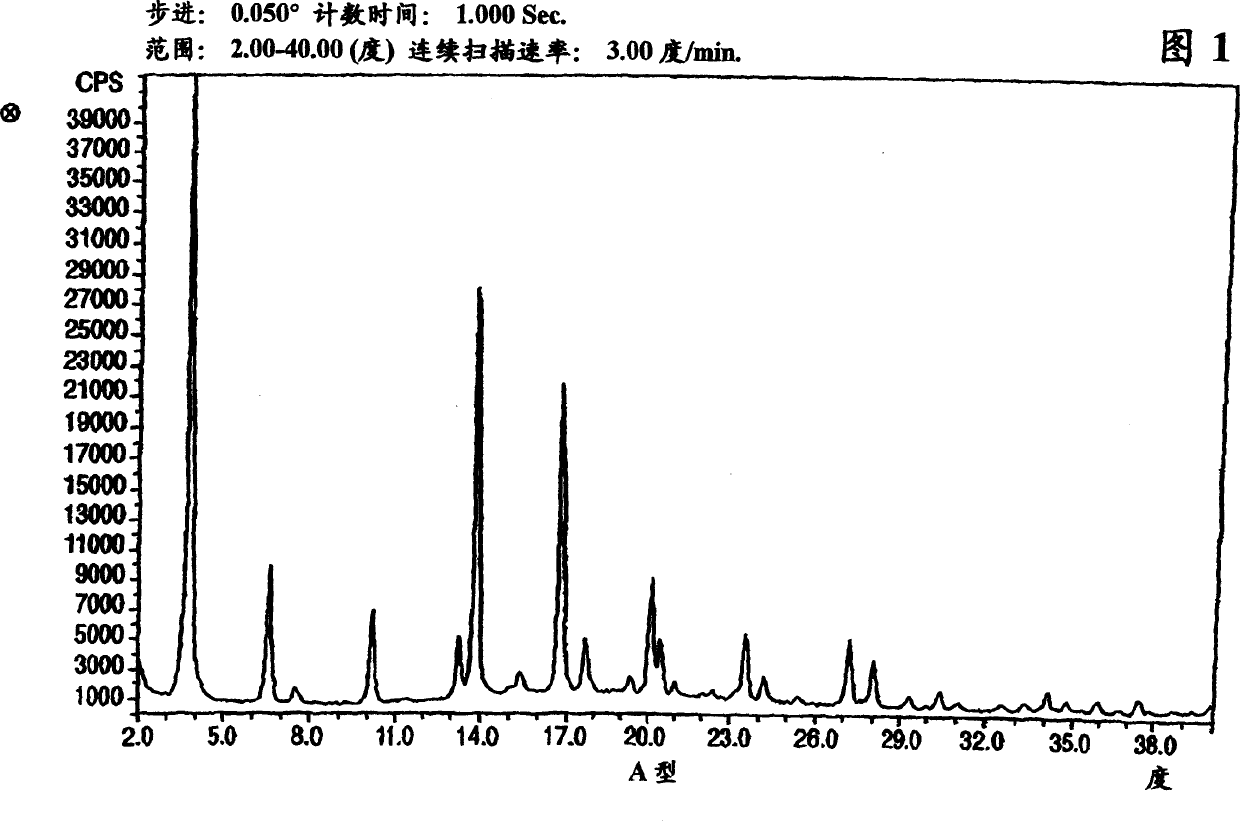
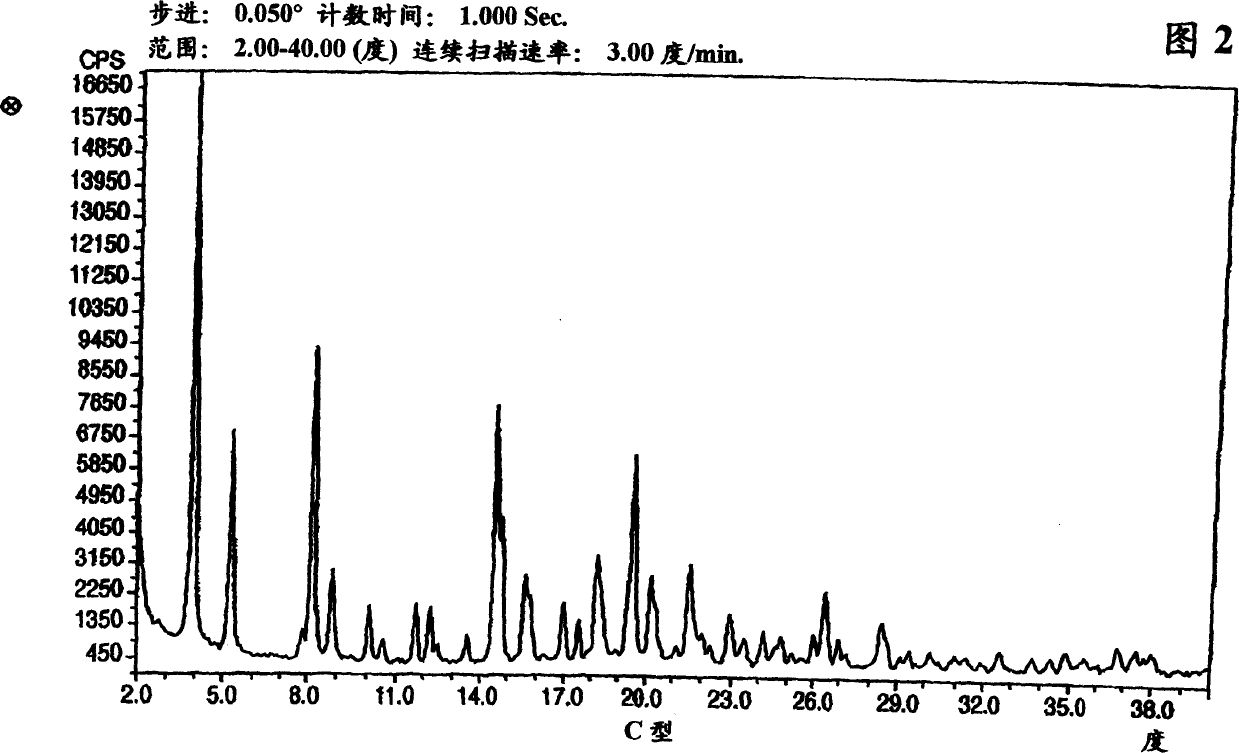
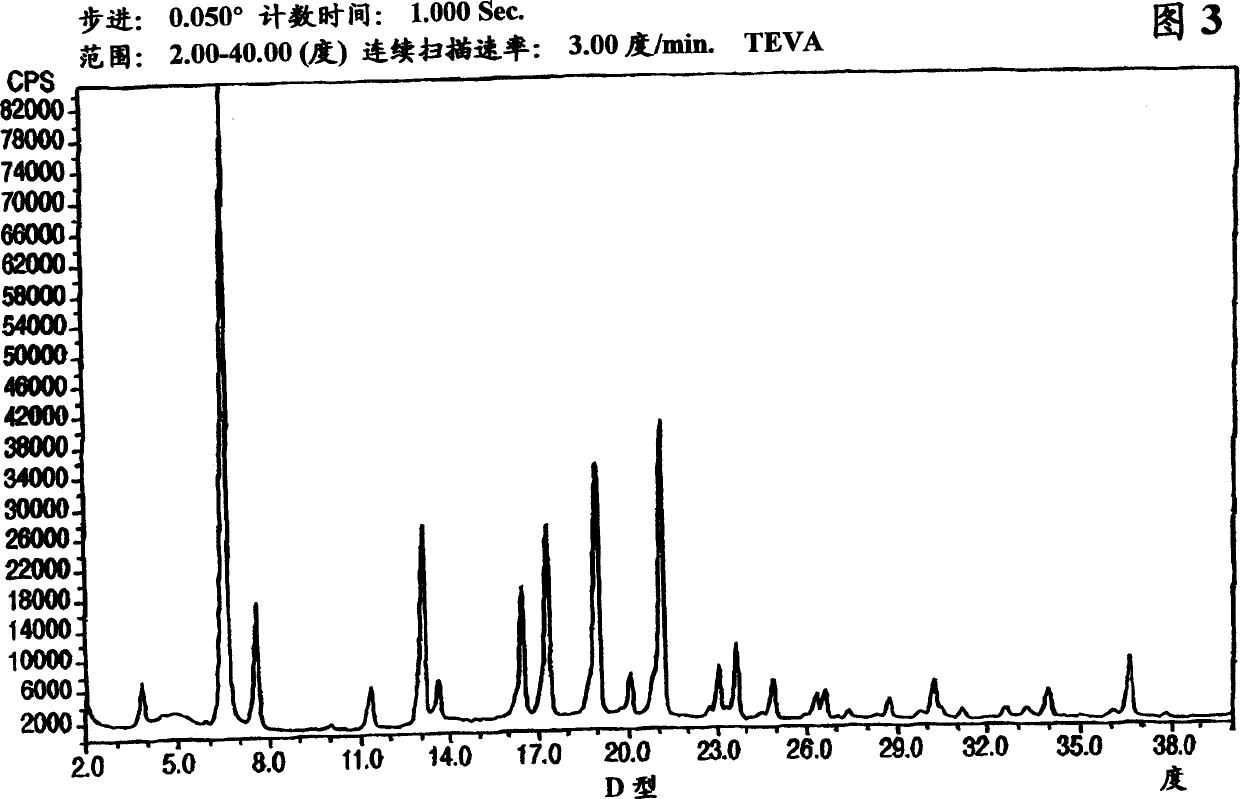
Polymorphic forms of nateglinide
A technology of nateglinide and crystal form, applied in the field of solid-state chemistry, can solve the problems of unstable and easy-to-change B-type crystals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0193] Example 1 - This example illustrates the preparation of various nateglinide crystal forms from solution
[0194] Nateglinide (5g) was placed in an Erlenmeyer flask and heated to a specific temperature. The solvent was added in 1 ml portions (in some cases in 5 ml portions) until a clear solution formed. If a clear solution did not result after addition of 150 ml of the solvent, the hot mixture was filtered.
[0195] The clear solution crystallized at room temperature. If no or very few crystals appeared, the solution was frozen at 3°C. The precipitate was filtered off (at room temperature or 5°C, depending on the crystallization temperature), weighed and divided into two equal portions. One portion was dried at 50°C under reduced pressure (20-30mmHg) for about 3-10 hours to constant weight (±0.01g). Details are listed in Table IV.
[0196] solvent
L / S,
ml / g
T,
℃
time
RT, h
time
3°C, h
crystal form
wet
...
Embodiment 2
[0200] Example 2 - This example illustrates the milling of Form H and U nateglinide in various solvents
[0201] Nateglinide (5g) was placed in an Erlenmeyer flask. The solvent was added in 1 ml portions to prepare a stirrable mixture. The bottle was stirred at room temperature with a magnetic stirrer. The solid was filtered off at room temperature, weighed, and divided into 2 equal portions. One portion was dried at 55°C under a pressure of 20-30mm / Hg to constant weight (±0.01g).
[0202] Details are listed in Tables V and VI.
[0203] start
[0204] start
Embodiment 3
[0205] Example 3 - This example illustrates the absorption of nateglinide to solvent vapor
[0206] Nateglinide (3.50 g) was added to a polypropylene jar and weighed. The jars were introduced into a larger polypropylene container containing solvent and stored at room temperature. The jar was removed from the container and weighed (Wfinal). The can contents are divided into 2 parts. One portion was dried to constant weight (±0.01 g) at 55°C under 20-30 mmHg pressure. Details are listed in Table VII.
[0207] NTGW,
[0208] Legend: Brutto - start weight of tank with NTG; Wfinal - final weight of tank with NTG after exposure; Δ - excess weight
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com