Method for separating nateglinide and its stereoisomers by high performance liquid chromatography
A technology of high performance liquid chromatography and stereoisomers, which is applied in the field of medicine, can solve the problems of high buffer concentration and short service life of chromatographic columns, and achieve high separation efficiency, guaranteed effectiveness and safety, and short separation time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
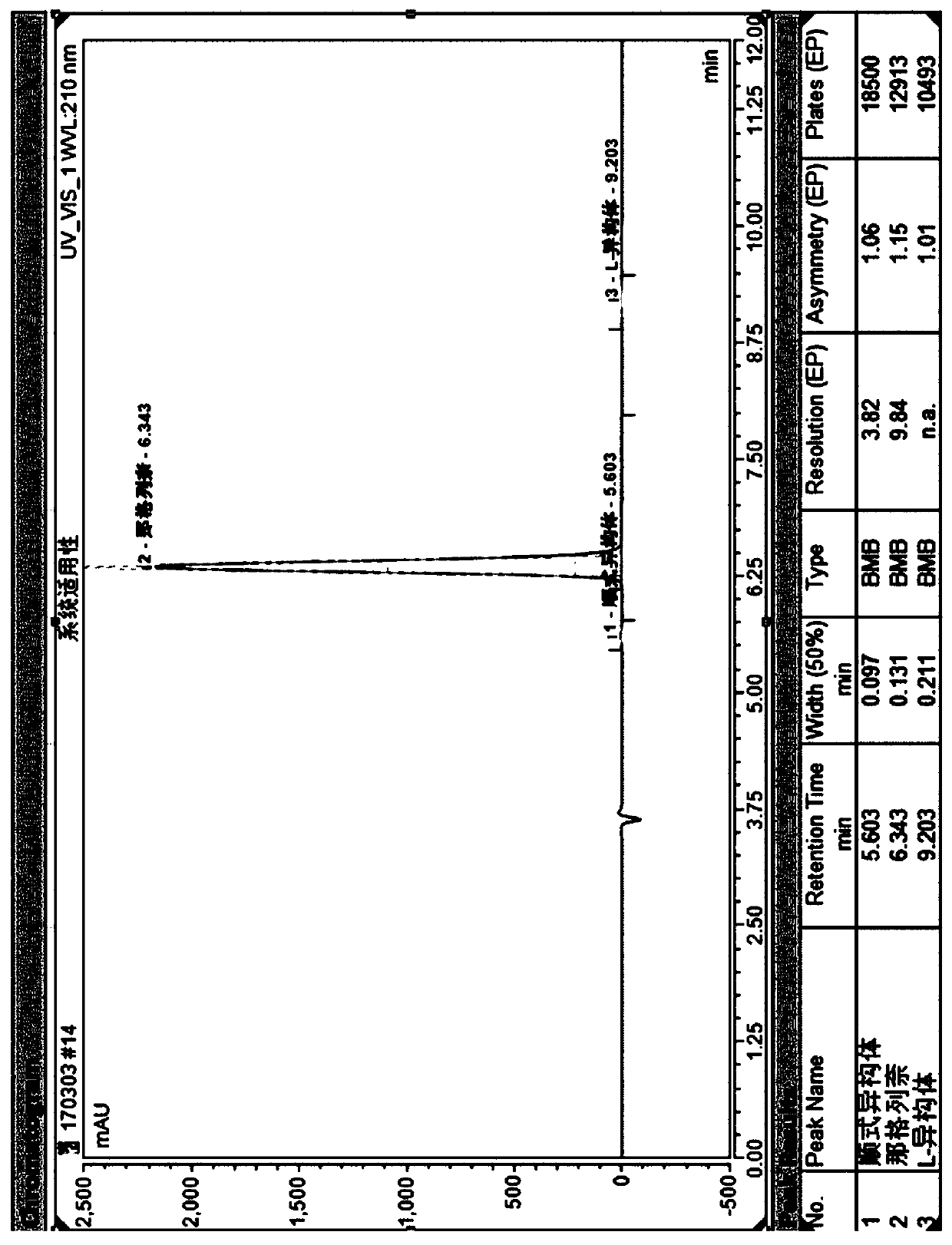
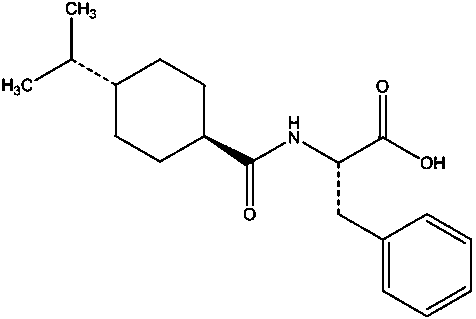
[0028] Example 1, a method for simultaneous resolution of nateglinide and its cis-isomer and L-isomer by high-performance liquid chromatography: Weigh nateglinide, cis-isomer and L-isomer respectively An appropriate amount of isomer, dissolved and diluted with n-hexane: absolute ethanol (80:20) to make a mixed solution containing about 1 mg of nateglinide, 2 µg of cis-isomer and 2 µg of L-isomer per 1 mL ;Amylose-tris(3-chloro-5-methylphenylcarbamate) covalently bonded to the surface of silica gel as the stationary phase, and a mixed solution of n-hexane, absolute ethanol and trifluoroacetic acid as the mobile phase , using a high-performance liquid chromatography system to resolve nateglinide and its isomers; the surface is covalently bonded with amylose-tris(3-chloro-5-methylphenylcarbamate) type The chiral column is bonded chiral column CHIRALPAK IG. The flow rate of the mobile phase is 1 mL / min, the temperature of the chromatographic column is 30° C., and the detection wa...
Embodiment 2
[0029] Example 2, a method for simultaneous resolution of nateglinide and its cis-isomer and L-isomer by high-performance liquid chromatography: Weigh nateglinide, cis-isomer and L-isomer respectively An appropriate amount of isomers was dissolved and diluted with n-hexane: absolute ethanol (75:25) to make a mixed solution containing about 1 mg of nateglinide, 2 µg of cis-isomers and 2 µg of L-isomers per 1 mL ;Amylose-tris(3-chloro-5-methylphenylcarbamate) covalently bonded to the surface of silica gel as the stationary phase, and a mixed solution of n-hexane, absolute ethanol and trifluoroacetic acid as the mobile phase , using a high-performance liquid chromatography system to resolve nateglinide and its isomers; the surface is covalently bonded with amylose-tris(3-chloro-5-methylphenylcarbamate) type The chiral column is bonded chiral column CHIRALPAK IG. The flow rate of the mobile phase is 0.8mL / min, the temperature of the chromatographic column is 30°C, and the detecti...
Embodiment 3
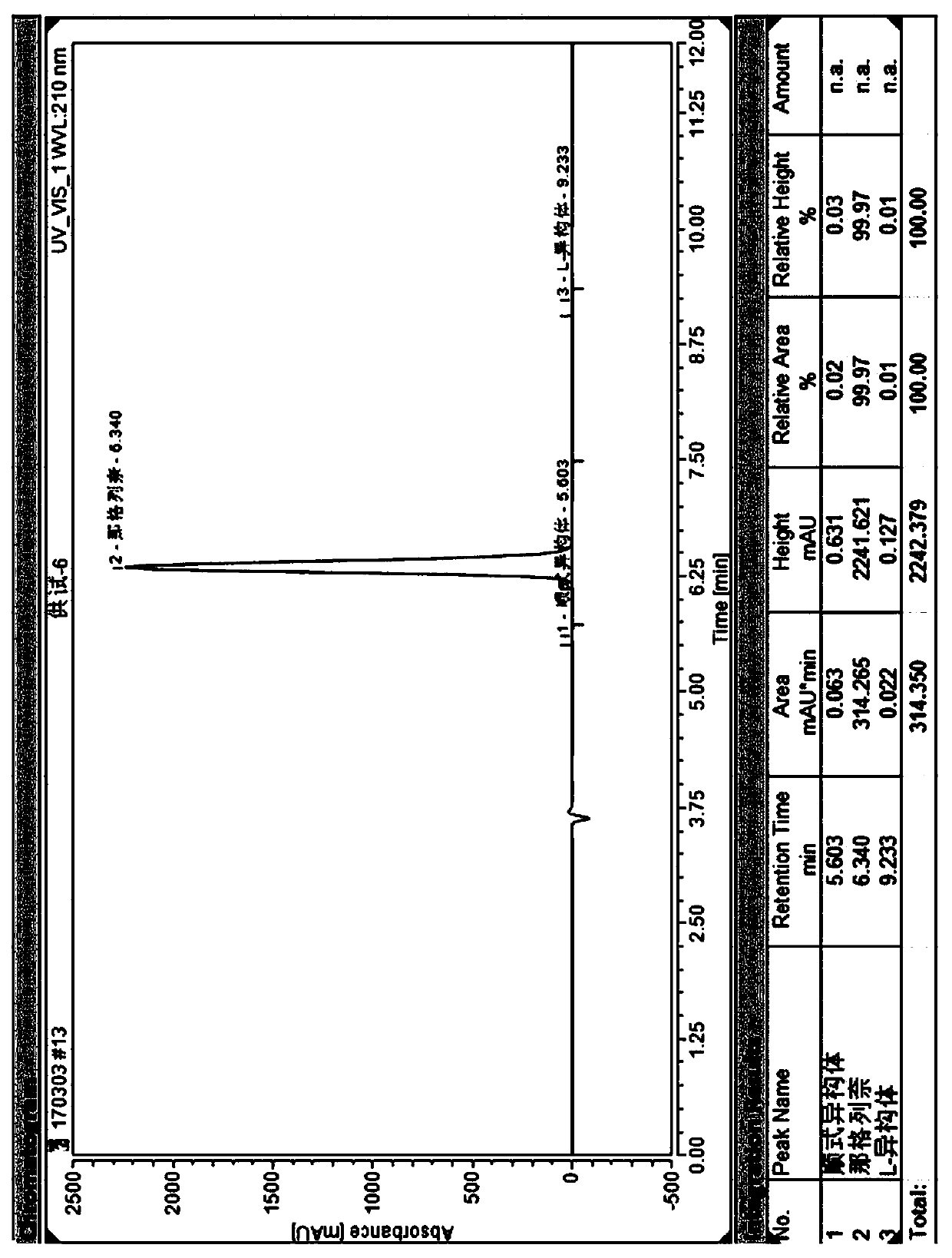
[0030] Example 3, a method for simultaneous resolution of nateglinide and its cis-isomer and L-isomer by high-performance liquid chromatography: Weigh nateglinide, cis-isomer and L-isomer respectively An appropriate amount of isomer, dissolved and diluted with an appropriate amount of diluent to make a mixed solution containing about 1 mg of nateglinide, 2 µg of cis-isomer and 2 µg of L-isomer per 1 mL; Amylose-tris(3-chloro-5-methylphenylcarbamate) was used as the stationary phase, and a mixed solution of n-hexane, absolute ethanol and trifluoroacetic acid was used as the mobile phase, and a high-performance liquid chromatography system was used for resolution Nateglinide and its isomers; the diluent is n-hexane: absolute ethanol (75:25). The flow rate of the mobile phase is 0.8mL / min, the temperature of the chromatographic column is 30°C, and the detection wavelength is 220nm. The injection volume is 20 μL. In the mobile phase, by volume ratio, n-hexane: absolute ethanol: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com