Solid compound of dapagliflozin as well as preparation method and application of solid compound
A complex, form of technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The preparation method of the co-crystal represented by formula II formed by dapagliflozin and nateglinide:
[0069] Test 1: Add nateglinide (776mg, 2.4mmol, 1.0eq) and dapagliflozin (1.0g, 2.4mmol, 1.0eq) to 4mL of ethyl acetate, stir to dissolve at room temperature, and slowly add 50mL of n-hexane dropwise After dripping, the temperature was slowly lowered to 0°C, a white solid was precipitated, suction filtered, the filter cake was rinsed with 5 mL of n-hexane, and then dried under vacuum at 40°C to obtain 1.5 g of a white solid with a yield of 84.5%.
[0070] Test 2: Add nateglinide (776mg, 2.4mmol, 1.0eq) and dapagliflozin (1.0g, 2.4mmol, 1.0eq) to 4mL of ethanol, stir to dissolve at room temperature, slowly add 30mL of water dropwise at 60°C, After dripping, the temperature was slowly lowered to 0°C to precipitate a white solid, which was filtered off with suction. The filter cake was rinsed with 5 mL of water, and then dried under vacuum at 40°C to obtain 1.62 g ...
Embodiment 2
[0081] The preparation method of the co-crystal formed by dapagliflozin and candesartan cilexetil of the present embodiment:
[0082] Preparation method of dapagliflozin and candesartan medoxomil co-crystal form I
[0083] Dapagliflozin (816mg, 2.0mmol, 1.0eq) and candesartan medoxomil (1.22g, 2.0mmol, 1.0eq) were added to a mixed system of 5mL of dichloromethane and 2mL of methanol, stirred at room temperature to dissolve, and then rotated The solvent was removed to obtain an oily substance, which was dissolved by adding 5 mL of ethyl acetate, and then slowly added dropwise with 40 mL of n-hexane. After drying, 1.95 g of white solid was obtained, which was identified as the co-crystal form I of dapagliflozin and candesartan cilexetil, and the yield was 96.1%.
[0084] The X-ray powder diffraction (XRPD) data of the co-crystal form I of dapagliflozin and candesartan cilexetil obtained in this example are shown in Table 2, and the XRPD pattern is shown in Table 2. Figure 4 ....
Embodiment 3
[0100] The preparation method of the solid amorphous formed by dapagliflozin and alalogliptin:
[0101] Alalogliptin (250 mg, 0.65 mmol, 1.0 eq) and dapagliflozin (266 mg, 0.65 mmol, 1.0 eq) were added to 10 mL of dichloromethane, stirred at room temperature to dissolve, and then spun off the solvent to obtain an oily substance, which was added 10 mL of n-hexane was slurried at room temperature, the oily substance turned into a white solid, filtered with suction, the filter cake was rinsed with 5 mL of n-hexane, and then dried under vacuum at 45°C to obtain 0.50 g of a white solid with a yield of 96.9%.
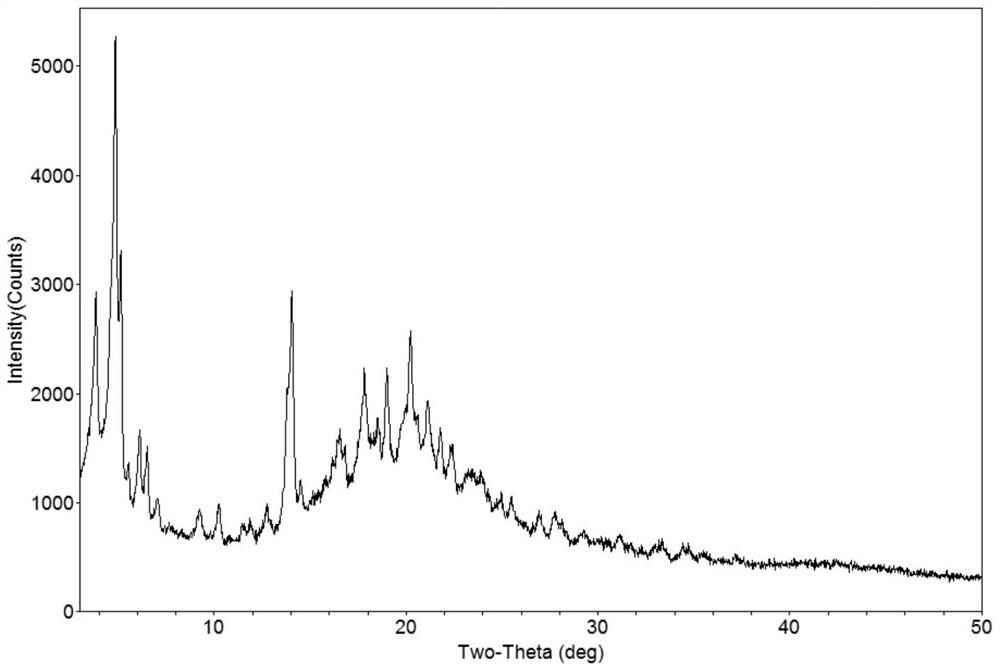
[0102] The XRPD diagram of the solid amorphous of dapagliflozin and alalogliptin obtained in this example is as follows Figure 11 , Figure 11 X-ray powder diffraction showed no characteristic peaks, indicating that the obtained complex was amorphous.
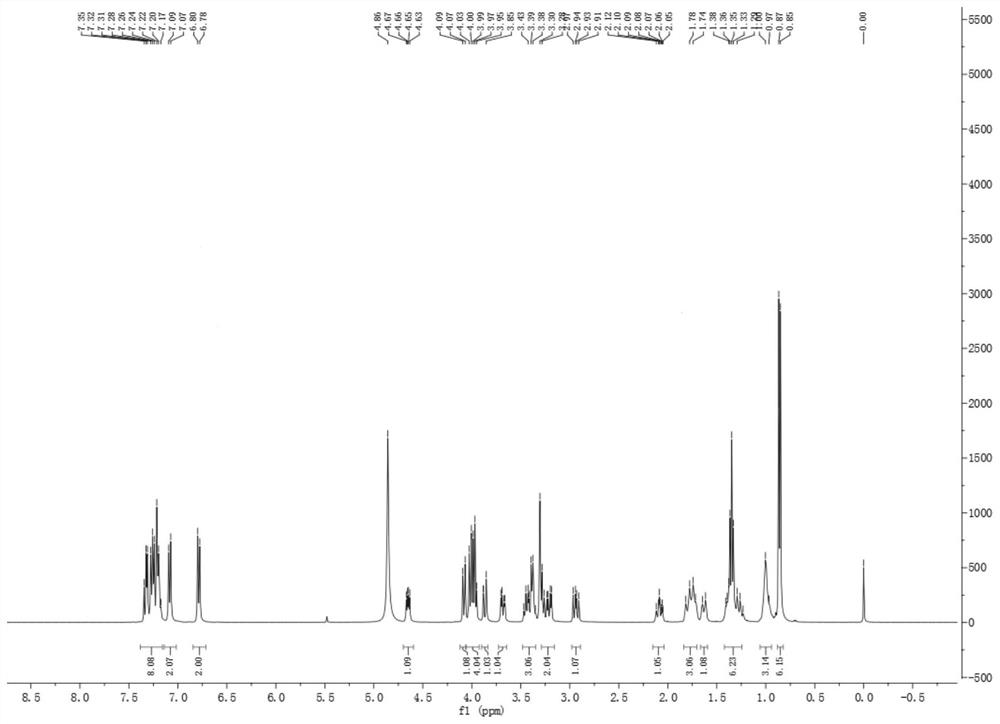
[0103] Nuclear Magnetic Resonance Spectroscopy 1 H-NMR as Figure 12 .
[0104] 1 H NMR (400MHz, MeOD) δ 9.33 (d, J=1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting range | aaaaa | aaaaa |
| Melting range | aaaaa | aaaaa |
| Melting range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com