Glipizide controlled release composition as well as preparation method thereof
A technology of glipizide and composition, applied in the field of glipizide controlled-release composition and its preparation, can solve problems such as organic solvent residues, avoid residues, reduce the probability of unqualified tablets, and be simple and easy to operate control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
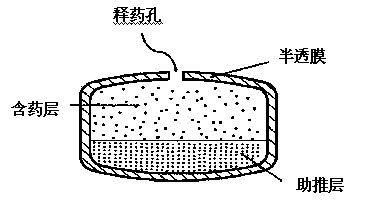
[0044] The preparation of the booster layer, the specific prescription components are as follows.
[0045]
[0046] i) Preparation of solutions and suspensions for granulation
[0047] ① Preparation of granulation suspension: Weigh purified water, add a small amount of binder (sodium carboxymethyl cellulose, hypromellose, hydroxypropyl cellulose, polyoxyethylene) while stirring, and stir until viscous The mixture is completely swollen, then add the prescribed amount of iron oxide red, stir to make the iron oxide red completely mix to form a suspension, pass through a 100-mesh sieve, then add normal temperature purified water, stir and mix, and set aside;
[0048] ② Preparation of binder solution: Weigh purified water, add remaining binder (sodium carboxymethyl cellulose, hypromellose, hydroxypropyl cellulose, polyoxyethylene) to the prescribed amount while stirring, and stir Until the adhesive is completely swollen, set aside;
Embodiment 2
[0057] The preparation of the drug-containing layer, the specific prescription components are as follows.
[0058]
[0059] i) Preparation of suspension for granulation
[0060] ① Preparation of binder solution: Weigh purified water, add the prescribed amount of binder (sodium carboxymethylcellulose, hypromellose, hydroxypropyl cellulose) while stirring, and stir until the binder is completely dissolved. up, spare;
[0061] ② Weigh the prescribed amount of adhesive solution in ①, and add the prescribed amount of glipizide to form a suspension while stirring. The suspension is homogenized under high pressure until the particle size of glipizide is less than 10 μm, and set aside;
[0062] ii) Weigh the prescription amount of polyoxyethylene and add it to the fluidized bed granulator, preheating;
[0063] iii) top spray granulation;
[0064] iv) After stopping the liquid spraying, dry, and the particles pass through a 24-mesh sieve;
[0065] v) Add the prescribed amount o...
Embodiment 3
[0082] Embodiment 3 Tablet comparative investigation
[0083] The granules in the prescription B of Example 1 and the prescription b of Example 2 and the mixed powders in Comparative Example 1 and Comparative Example 2 were selected respectively, and the same double-layer tablet press was used to compress the double-layer tablet with the same parameters, and the results are shown in the following table shown.
[0084] Numbering booster layer Drug-containing layer result Ⅰ Embodiment 1 prescription B Embodiment 2 prescription b There is no mixture of two layers of color, and almost no flower flakes are found. Ⅱ Comparative example 1 Comparative example 2 The colors are mixed, and the flower flakes are obvious. Ⅲ Comparative example 1 Embodiment 2 prescription b There are red spots and plaques on the surface of the white layer. Ⅳ Embodiment 1 prescription B Comparative example 2 There are white spots on the surface of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com