Glipizide tablets for treating diabetes and preparation method thereof
A technology of glipizide tablets and glipizide, which is applied to chemical instruments and methods, metabolic diseases, mixers with rotating stirring devices, etc., can solve the problems of unsteady dissolution and other problems, and achieve simple structure, stable release, The effect of convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Weigh 10g of glipizide, 30g of hydroxypropyl methylcellulose, 40g of calcium carbonate, 15g of L-aspartic acid, 15g of sodium alginate, 20g of calcium sulfate dihydrate, 10g of guar gum, and crospovidone Ketone 8g, magnesium stearate 1g.
[0018] Preparation process: put the glipizide raw material drug in a crushing equipment and pulverize it; mix the pulverized glipizide raw material drug and the above-mentioned excipients in a particle mixer according to the amount, granulate according to a conventional method, compress tablets, and pack to obtain finished product.
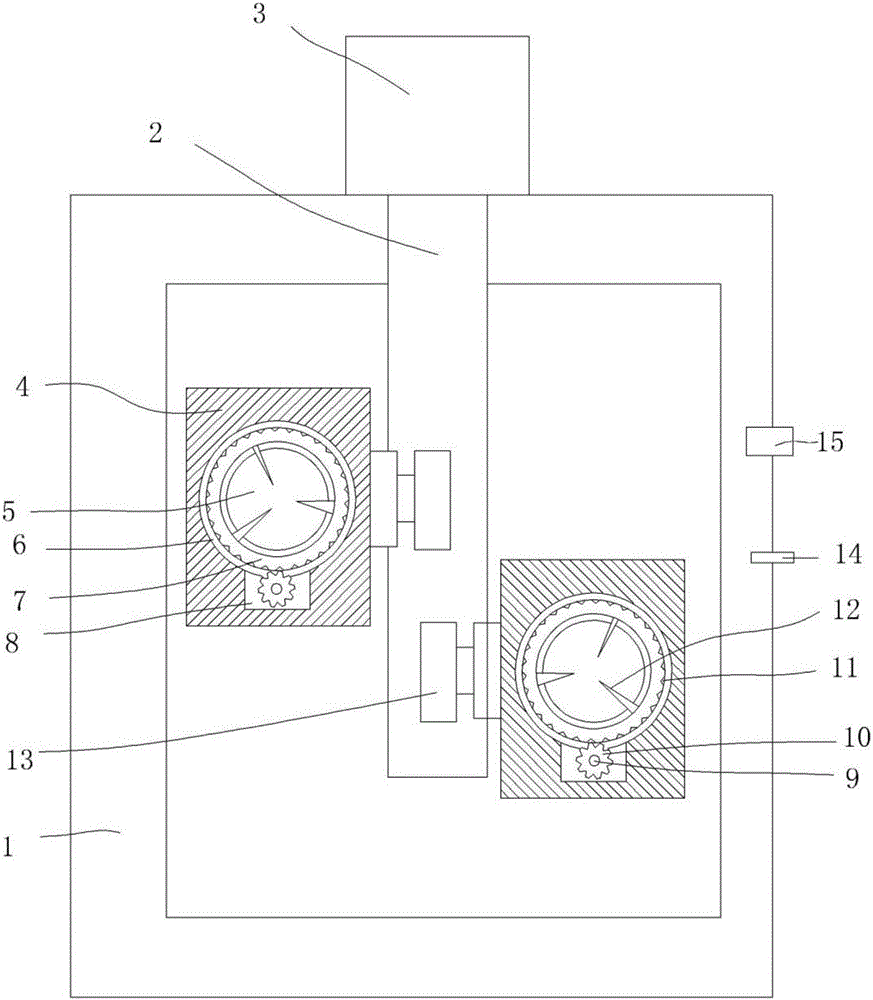
[0019] Particle mixers above, see figure 1 , comprising a stirring barrel 1, a stirring shaft 2 arranged in the stirring barrel 1 and a stirring motor 3 arranged on the stirring barrel 1 and connected with the stirring shaft 2, and the stirring shaft 2 is provided with a stirring The paddle 4 is provided with a through hole 5 on the stirring paddle 4, an annular groove 6 is provided on the inner wall of...
Embodiment 5
[0032] The release research of embodiment 5 glipizide tablet
[0033] Get respectively the tablet that the embodiment of the present invention 1, comparative example 2-4 prepare, according to the release assay method [2005 version 5 Chinese Pharmacopoeia (two) appendix XD first method], with 0.5% sodium lauryl sulfate 1000mL of the solution is used as the solvent, and the rotation speed is 50r / min. Operate according to the law. Take 10mL of the solution at 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 hours, filter, and replenish the above Solvent 10mL; Get continued filtrate, according to spectrophotometry (2005 edition 5 Chinese Pharmacopoeia (two) appendix IVA) measure absorbance at 274nm wavelength place; Separately accurately weigh the glipizide reference substance amount of drying 2h through 105 degree, add 0.5% sodium dodecyl sulfate solution was heated and ultrasonically dissolved and diluted quantitatively to a solution containing 10ug of drug per 1mL. The absorbance...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com