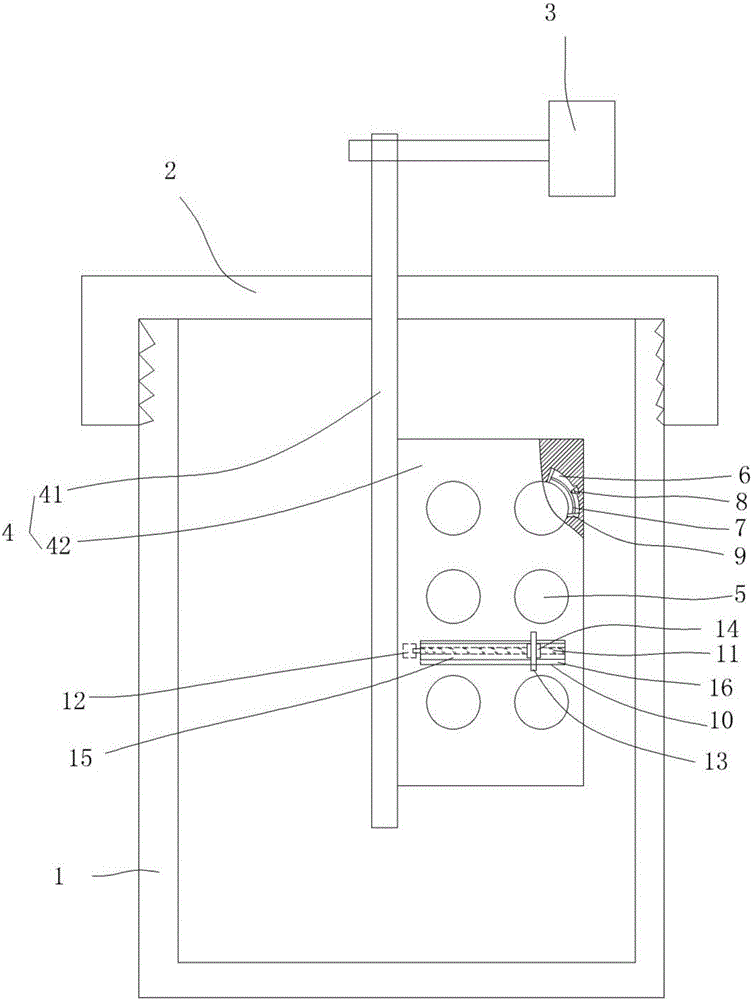
Theophylline sustained-release tablet for treating bronchial asthma and method for preparing theophylline sustained-release tablet
A technology for bronchial asthma and sustained-release tablets, which is applied to chemical instruments and methods, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of unstable release of theophylline tablets and large fluctuations in blood drug concentration , Inconvenient for patients to take and other problems, achieve simple structure, stable release, and solve the effect of uneven stirring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Take the weight of the following raw and auxiliary materials in proportion, and in terms of the total weight percentage of the sustained-release tablet, the plain tablet is composed of the following ingredients: 50 parts of theophylline, 20 parts of L-alanine, 20 parts of polyvinylpyrrolidone, stearin Magnesium acid 5 parts, alginic acid 8 parts and lactose 10 parts. The weight formula ratio of the coating is as follows: 2 parts of sodium carboxymethylcellulose, 2 parts of propylene glycol, and 1 part of talcum powder.
[0021] Preparation Process:
[0022] (1) Dissolving polyvinylpyrrolidone in an ethanol solution to prepare an alcoholic solution; (2) putting the sieved theophylline, remaining polyvinylpyrrolidone, L-alanine, alginic acid, and lactose in a particle mixer (3) Add the lubricating glidant magnesium stearate after drying the above-mentioned soft material to obtain a mixed powder, and add all the mixed powder To a motion mixer, use a rotary lozenge machin...
Embodiment 5
[0036] The Release Research of Embodiment 5 Theophylline Sustained-release Tablets
[0037]Dissolution test method: get respectively embodiment 1 of the present invention, comparative example 2,3,4 to compare, according to the release determination method (two appendix XD first methods of Chinese Pharmacopoeia version in 2010), appendix of Chinese Pharmacopoeia version in 2010, blue The method is 75 revolutions / min, and 1000ml of fresh water with air removed is used as the release solvent for this product. Select UV spectrophotometry at 233nm to determine the release rate of theophylline sustained-release tablets by absorption coefficient method.
[0038] The sustained-release tablet release test result prepared by each embodiment of table 1
[0039] Sample source
[0040] According to the test results of Table 1, it can be seen that the theophylline sustained-release tablet prepared by Example 1 of the present invention releases smoothly; in comparative example 2-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com