Slow controlled released combsn. preparation of semisolid framework of containing glipizide
A technology of glipizide and semi-solid, which is applied in the field of sustained and controlled release semi-solid matrix preparation compositions, and can solve the problems of high incidence of toxic and side effects, frequent administration, and inability to release drugs stably for a long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
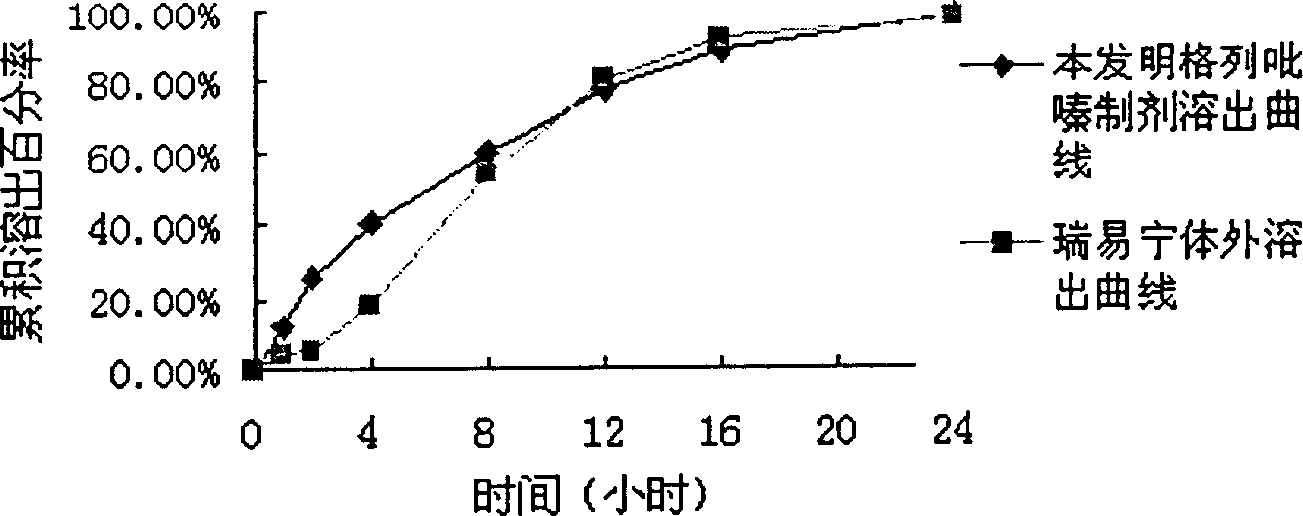
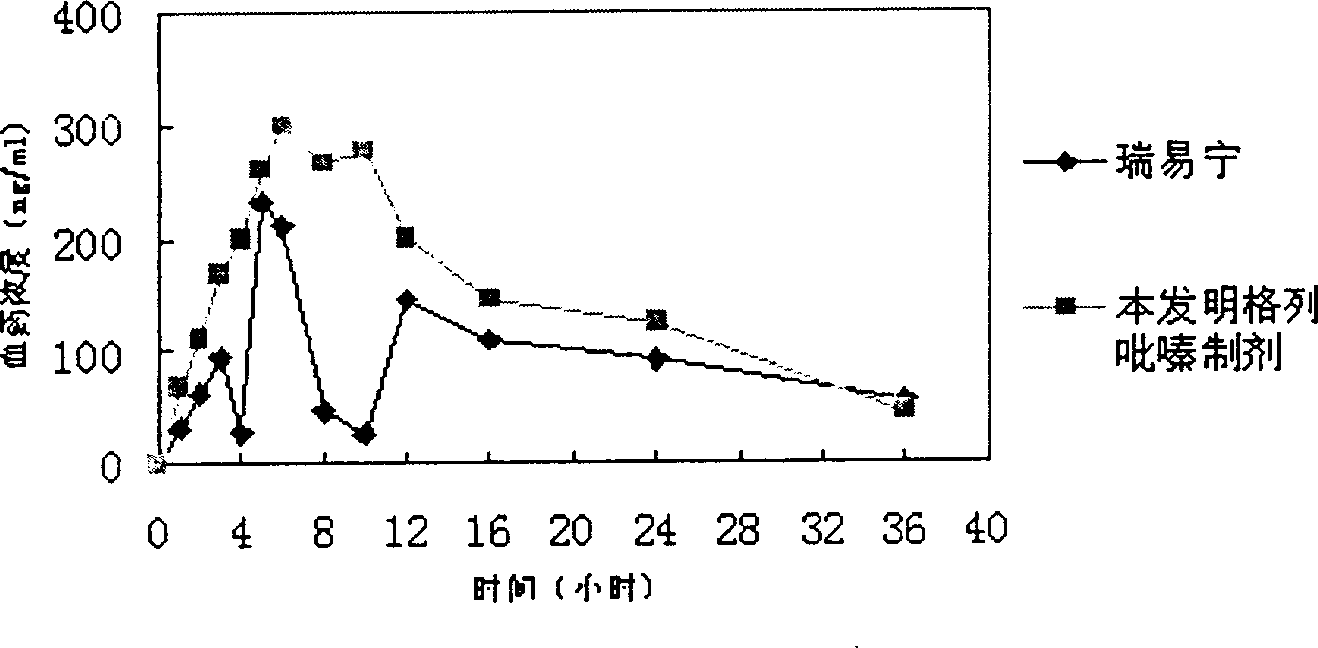
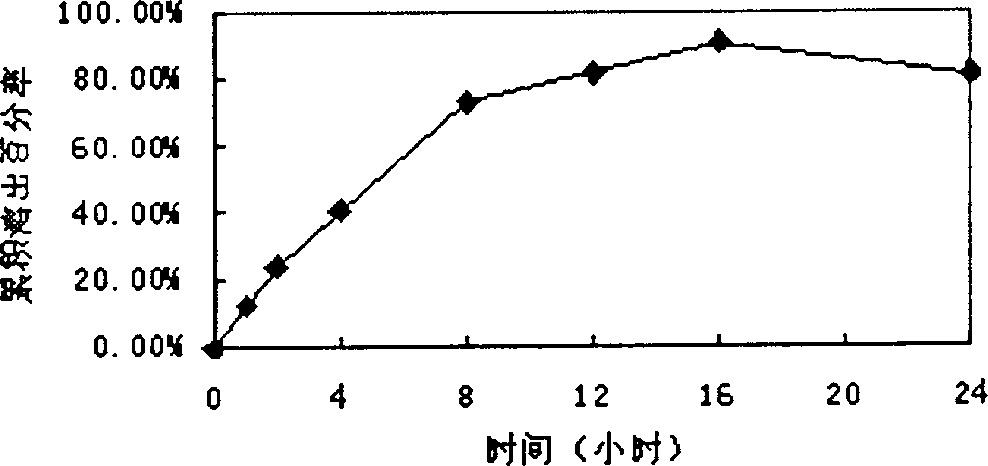
[0044]329g of macrogol glyceride stearate and 141g of glyceryl behenate, heat and melt at 80°C, add 5g of glipizide, stir to mix evenly, cool to 60-70°C and fill with capsule liquid Machine filling in No. 1 capsules to make 1000 capsules. In vitro release curve see image 3 shown. (Release 24H)
Embodiment 2
[0046] Glipizide 5, palmitic acid 200g, glyceryl behenate 150g, beeswax 150g, grind until fully dissolved, mix well and heat to 80°C, stir to mix evenly, cool to 60-70°C and use capsule liquid filling machine Fill in No. 1 capsules to obtain 1000 capsules. In vitro release curve see Figure 4 shown. (Release 24H)
Embodiment 3
[0048] 103.4g of macrogol glyceride stearate, 117.5g of carnauba wax, heat and melt at 80°C, add 5g of glipizide, 4.7g of ethyl cellulose (10CP), stir to make the mixture uniform, and slowly add 239.7g of soybean oil, after mixing, was cooled to 60-70°C and filled in No. 1 capsules with a capsule liquid filling machine to obtain 1000 capsules. In vitro release curve see Figure 5 shown. (Release 24H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com