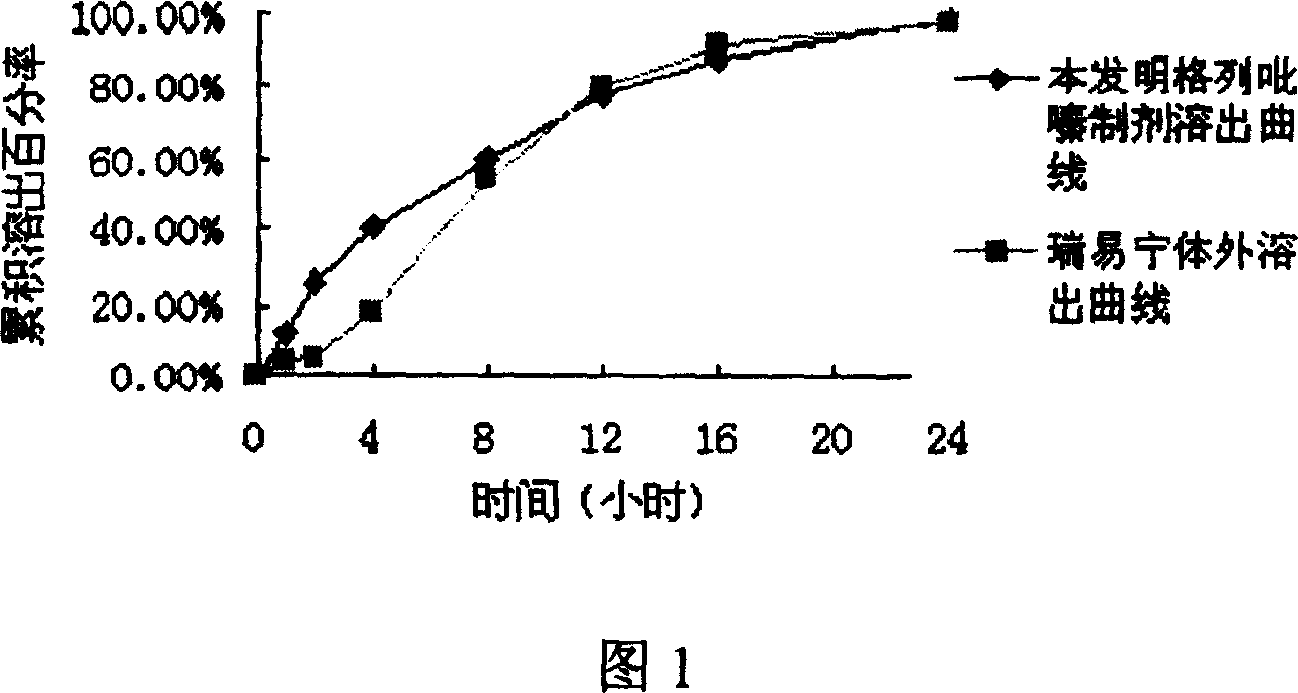
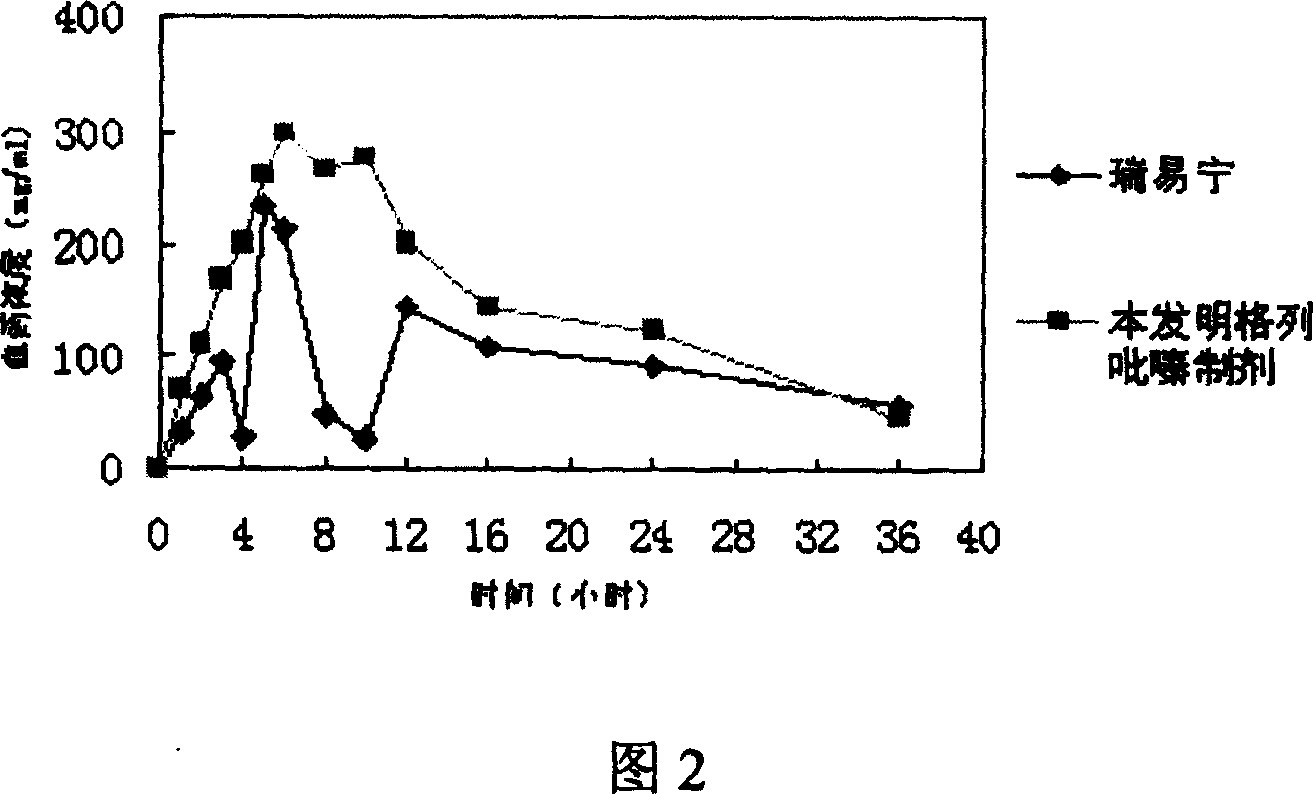
Slow controlled released combsn. preparation of semisolid framework of containing glipizide
A technology of glipizide and semi-solid, which is applied in the field of sustained and controlled release semi-solid matrix preparation composition, which can solve the problems of frequent administration, inability to release drug stably for a long time, and high incidence of toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
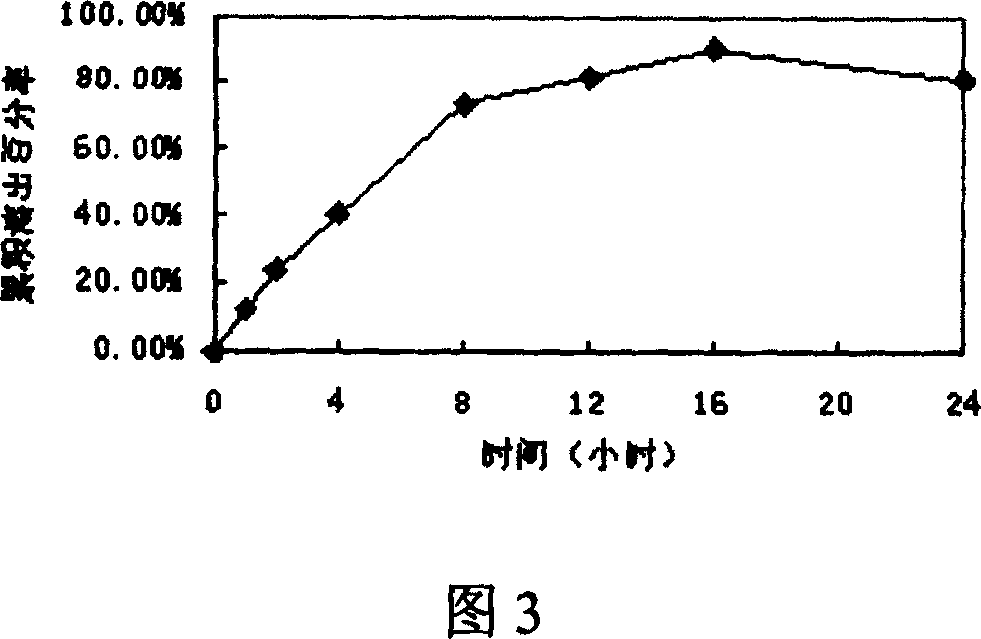
[0044] 329g of macrogol glyceride stearate and 141g of glyceryl behenate, heat and melt at 80°C, add 5g of glipizide, stir to mix evenly, cool to 60-70°C and fill with capsule liquid Machine filling in No. 1 capsules to make 1000 capsules. The in vitro release curve is shown in Figure 3. (Release 24H)
Embodiment 2
[0046] Glipizide 5, palmitic acid 200g, glyceryl behenate 150g, beeswax 150g, grind until fully dissolved, mix well and heat to 80°C, stir to mix evenly, cool to 60-70°C and use capsule liquid filling machine Fill in No. 1 capsules to obtain 1000 capsules. The in vitro release curve is shown in Figure 4. (release 24H)
Embodiment 3
[0048] 103.4g of macrogol glyceride stearate, 117.5g of carnauba wax, heat and melt at 80°C, add 5g of glipizide, 4.7g of ethyl cellulose (10CP), stir to make the mixture uniform, and slowly add 239.7 g of soybean oil, after mixing, was cooled to 60-70 DEG C and filled in No. 1 capsules with a capsule liquid filling machine to obtain 1000 capsules. The in vitro release curve is shown in Figure 5. (release 24H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com