Glipizide osmotic pump type controlled release tablet
A technology of glipizide and osmotic pumps, which is applied in medical preparations of non-active ingredients, pill delivery, metabolic diseases, etc., can solve the problems of increased residual drug release at the end, decreased release rate, and decreased membrane weight loss, etc. Achieve the effects of less drug release residue, lower deformability requirements, and lower process costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1, commercially available glipizide sustained-release tablet
[0067] Manufacturer: Pfizer Inc. Product Name: Ruiyining
[0068] Batch number: 85807012
[0069] Specification: 5mg
[0070] Release test: According to the import drug standard JX19990091 of the People's Republic of China, the specific method is as follows:
[0071] Get this product, according to the release assay method (Chinese Pharmacopoeia version two appendix XD in 1995, the first method), adopt the second method device of the dissolution assay method, with 900ml of artificial intestinal juice not containing pancreatin as solvent, the rotating speed is per minute 50 turns, operate in accordance with the law, get solution 8ml respectively when 4, 8 and 16 hours, filter, and supplement above-mentioned solvent 8ml in operating container clock immediately; Get continued filtrate, according to spectrophotometry (two appendix IV A ), respectively measure the absorbance at the wavelength of 27...
Embodiment 2
[0098] Embodiment 2 adopts ethyl cellulose and povidone to make the glipizide controlled-release tablet of film-forming material
[0099] 1. Prescription
[0100] 1. Tablet core prescription:
[0101] 1000 pieces, see table 4 below:
[0102] Table 4 embodiment 2 tablet core prescription
[0103]
[0104] 2. Prescription of coating solution: see Table 5 below:
[0105] Table 5 embodiment 2 coating solution prescription
[0106] Ethyl cellulose
30g
povidone k30
14g
950ml
water
50ml
[0107] Second, the preparation process:
[0108] 1. Tablet core preparation process:
[0109] (1) Glipizide passes through a 100-mesh sieve;
[0110] (2) Take lactose, sodium chloride, sodium lauryl sulfate, sodium carboxymethylcellulose, povidone K30 and mix according to the prescription amount of the drug-containing layer;
[0111] (3) make soft material with 10% povidone k30 85% ethanol solution;
[0112] (4) Pass throug...
Embodiment 3
[0136] Embodiment 3 adopts ethyl cellulose and polyethylene glycol to make the glipizide controlled-release tablet of film-forming material
[0137] 1. Prescription
[0138] 1. Tablet core prescription: same as Example 2.
[0139] 2. The composition of the coating solution prescription: see Table 9 below:
[0140] Table 9 embodiment 3 coating solution prescription
[0141] Ethylcellulose N-100
30g
Polyethylene glycol-4000
15g
800ml
water
200ml
[0142] Second, the preparation process:
[0143] 1, tablet core preparation process: with embodiment 2.
[0144] 2. The preparation process of the semi-permeable membrane coating solution is as follows:
[0145] Weigh the prescribed amount of ethyl cellulose and polyethylene glycol-4000 and disperse in the ethanol-water solution; stir to dissolve completely.
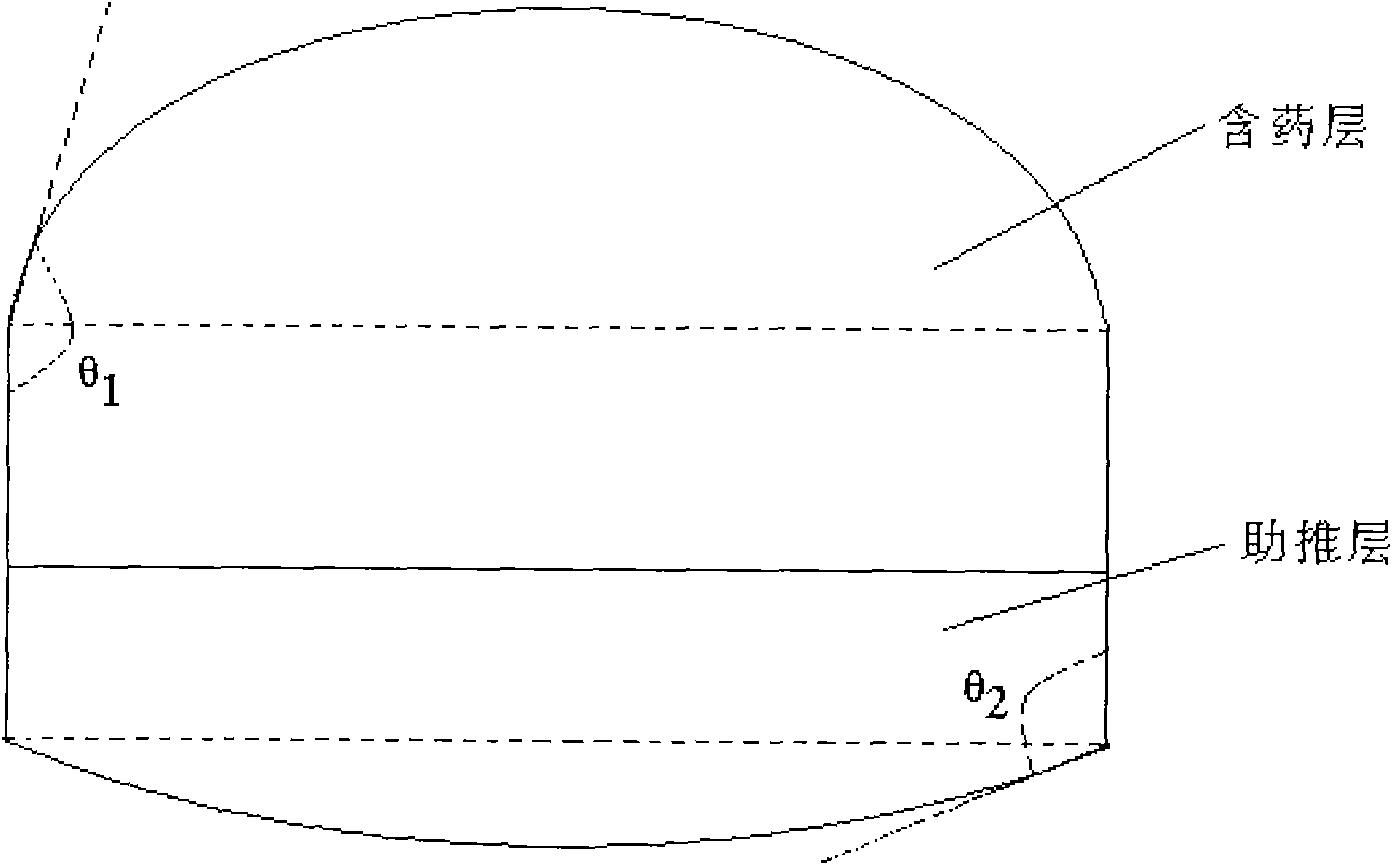
[0146] 3. Weight gain of semi-permeable membrane coating: 13.5%.
[0147] 4. Heat treatment: 40°C for 14 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com