Glipizide osmotic pump controlled release tablet
The technology of osmotic pump controlled release and glipizide is applied in the field of osmotic pump controlled release tablets and their preparation, and can solve the problem that the zero-order drug release characteristics of gel matrix sustained-release tablets are not obvious, the influence of drug stability, and the influence of drugs Release and other issues, to achieve the effect of easy coating control, good drug stability, and good controlled release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The prescription is as follows:
[0055] (1) Drug layer (per tablet):
[0056]
[0057] (2) Push layer (per piece):
[0058]
[0059] (3) Composition of semi-permeable membrane coating solution (for every 1000 tablets)
[0060] Cellulose acetate 70g
[0061] Diethyl phthalate 3.0g
[0062] Acetone: water (95:5) 2000m1
[0063] (4) Moisture-proof coating solution composition:
[0064] OPADRY II White(85668918) Appropriate amount
[0065] Preparation Process:
[0066] 1. Preparation of Drug-Layered Particles:
[0067] Mix the prescribed amount of glipizide, yellow iron oxide, sodium alginate and HPMC (K4M) uniformly by equal volume addition method, mix them in a mixer, add 75% ethanol after mixing uniformly to prepare a soft material, 20 mesh Sieve and granulate, dry at 50°C for more than 4 hours, measure moisture and drug content, then add magnesium stearate, and mix well.
[0068] 2. Preparation of push layer particles:
[0069] After passing through a 6...
Embodiment 2
[0078] (1) Drug layer (per tablet):
[0079]
[0080]
[0081] (2) Push layer (per piece):
[0082]
[0083] (3) Semi-permeable membrane coating
[0084]
[0085] The moisture-proof film coating and preparation process are the same as in Example 1.
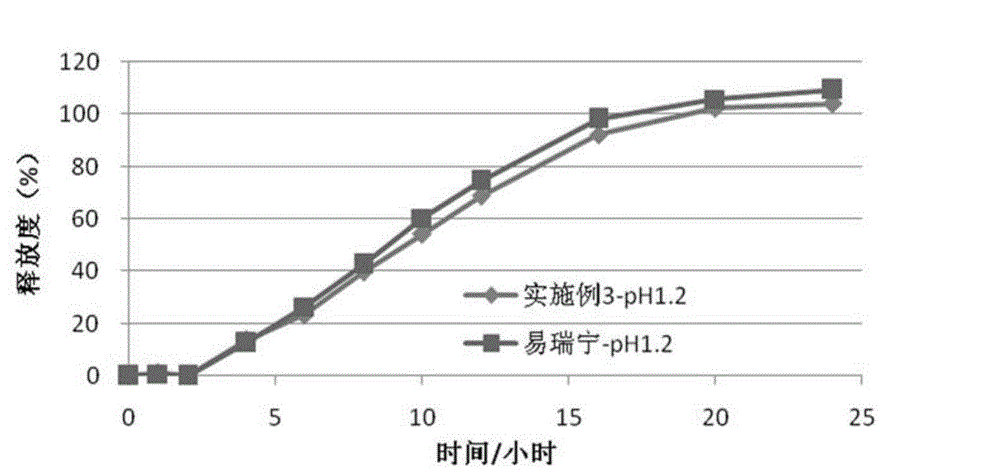
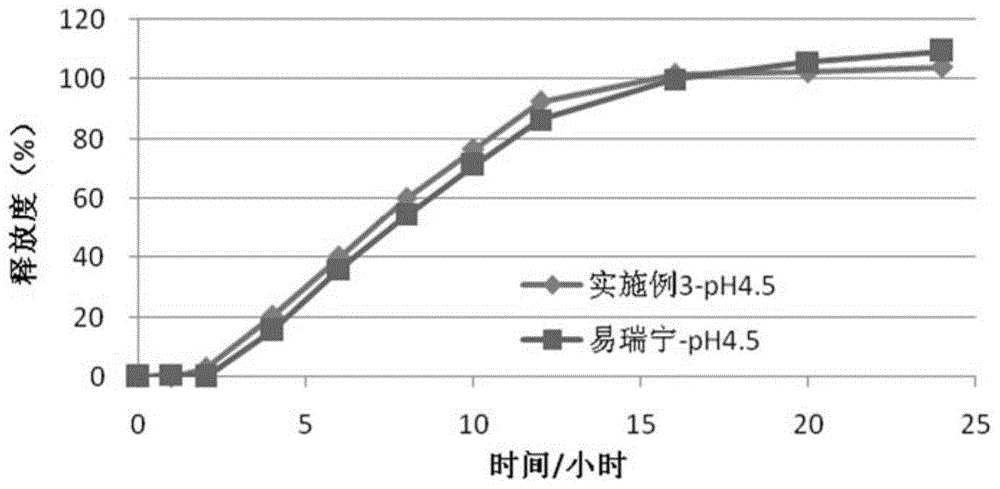
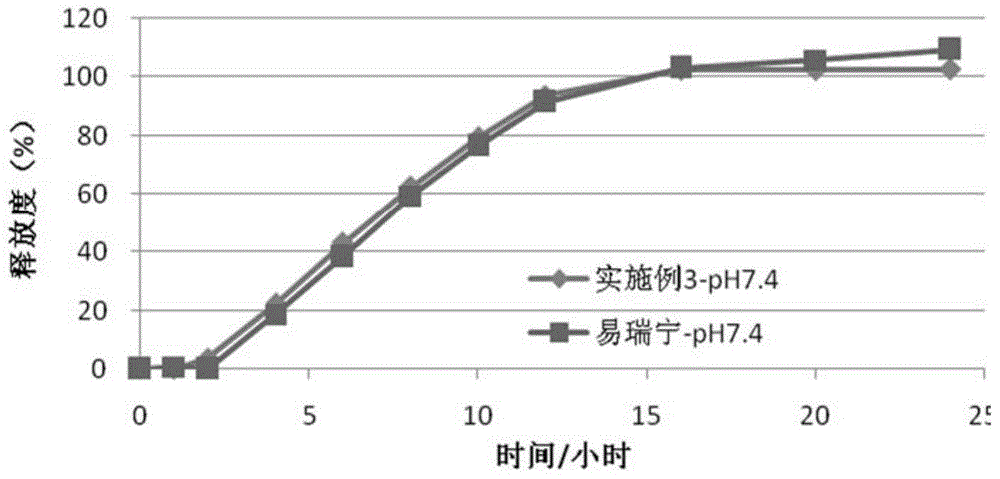
Embodiment 3
[0087] (1) Drug layer (per tablet):
[0088]
[0089] (2) Push layer (per piece):
[0090]
[0091]
[0092] (3) Semi-permeable membrane coating
[0093] Ethylcellulose 45g
[0094] Polyethylene glycol (4000) 6g
[0095] Ethanol 1000ml
[0096] The moisture-proof film coating and preparation process are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Film diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com