Risperidone slow-release microsphere, preparation method and application thereof
A technology of slow-release microspheres and risperidone, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve the problems of low drug loading and burst release, and achieve Improve compliance, maintain blood drug concentration, and reduce adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
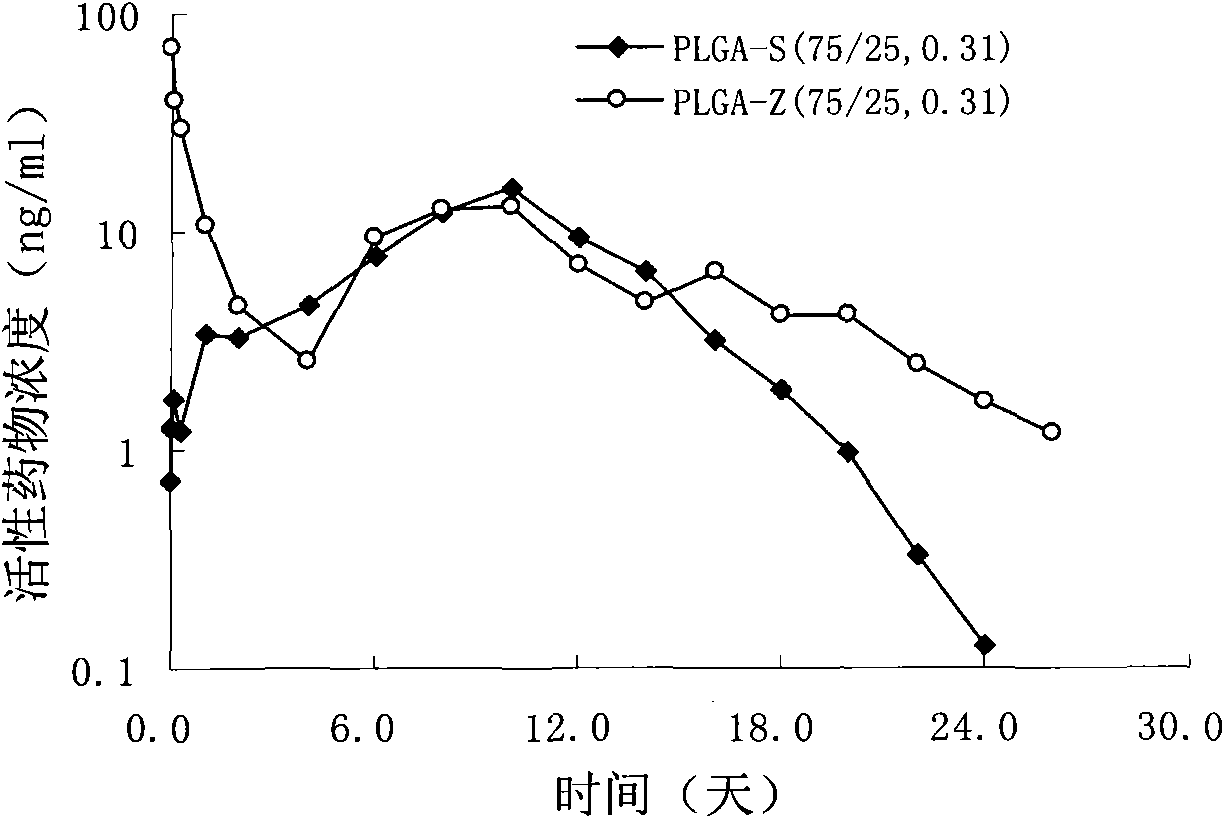
[0035] Weigh 4.0g PLGA-S (75 / 25, 0.31) molecular weight 33,000, 6.0g risperidone, add to 50ml of dichloromethane and stir to dissolve to obtain a clear solution, this solution is added to a high-speed stirring cooling to 6 In 5000ml PVA solution (0.5%) at ℃, disperse and emulsify at 1000rpm for 1min, adjust the rotation speed to 300rpm, and the rotation speed of the stirring paddle is 150rpm, volatilize to remove the organic solvent, and volatilize for 6hr; filter with a sieve, wash with deionized water 5 times, freeze The powdered microspheres were dried, with a drug loading of 49.4% and an encapsulation efficiency of 82.3%.
Embodiment 2
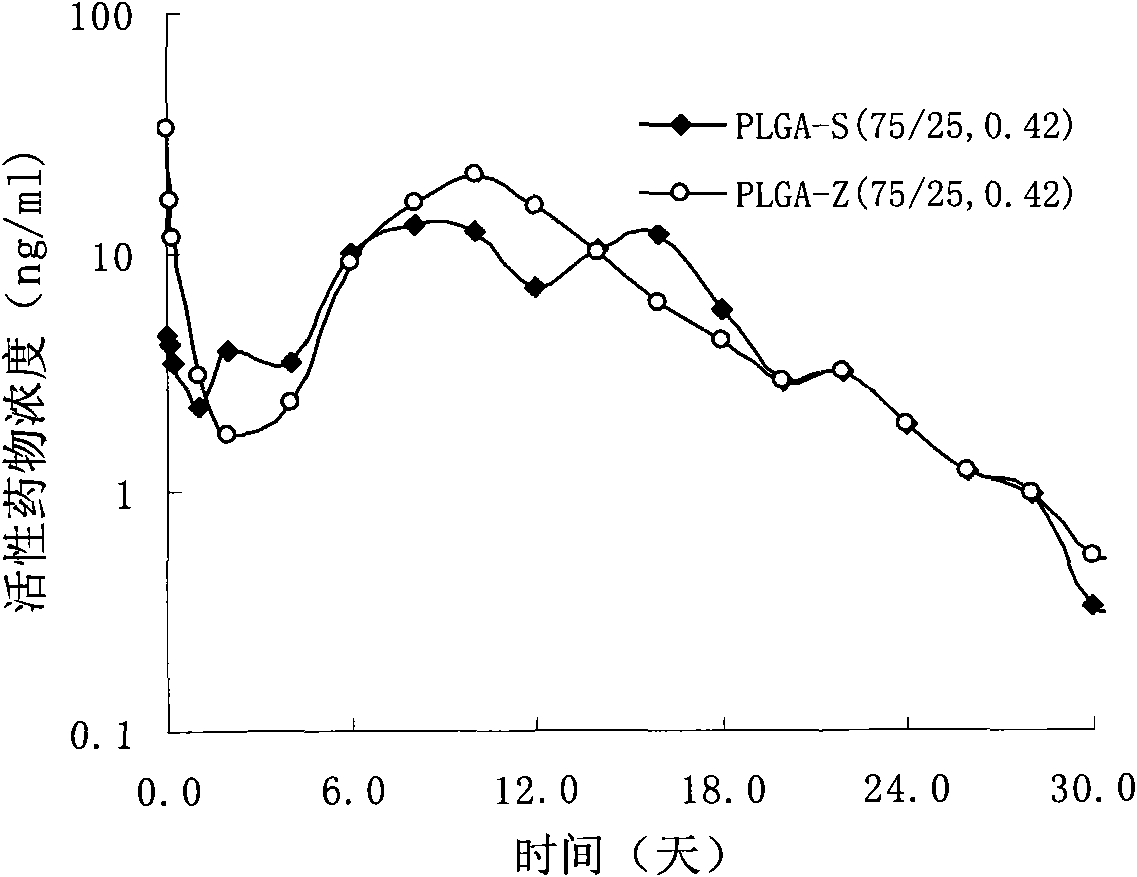
[0037] Weigh 4.0g PLGA-S (75 / 25, 0.42) molecular weight 53,000, 6.0g risperidone, add to 50ml of dichloromethane and stir to dissolve to obtain a clear solution, this solution is added to a high-speed stirring cooling to 6 In 5000ml PVA solution (0.5%) at ℃, disperse and emulsify at 1000rpm for 1min, adjust the rotation speed to 300rpm, and the rotation speed of the stirring paddle is 150rpm, volatilize to remove the organic solvent, and volatilize for 6hr; filter with a sieve, wash with deionized water 5 times, freeze The powdered microspheres were dried, with a drug loading of 49.0% and an encapsulation efficiency of 81.7%.
Embodiment 3
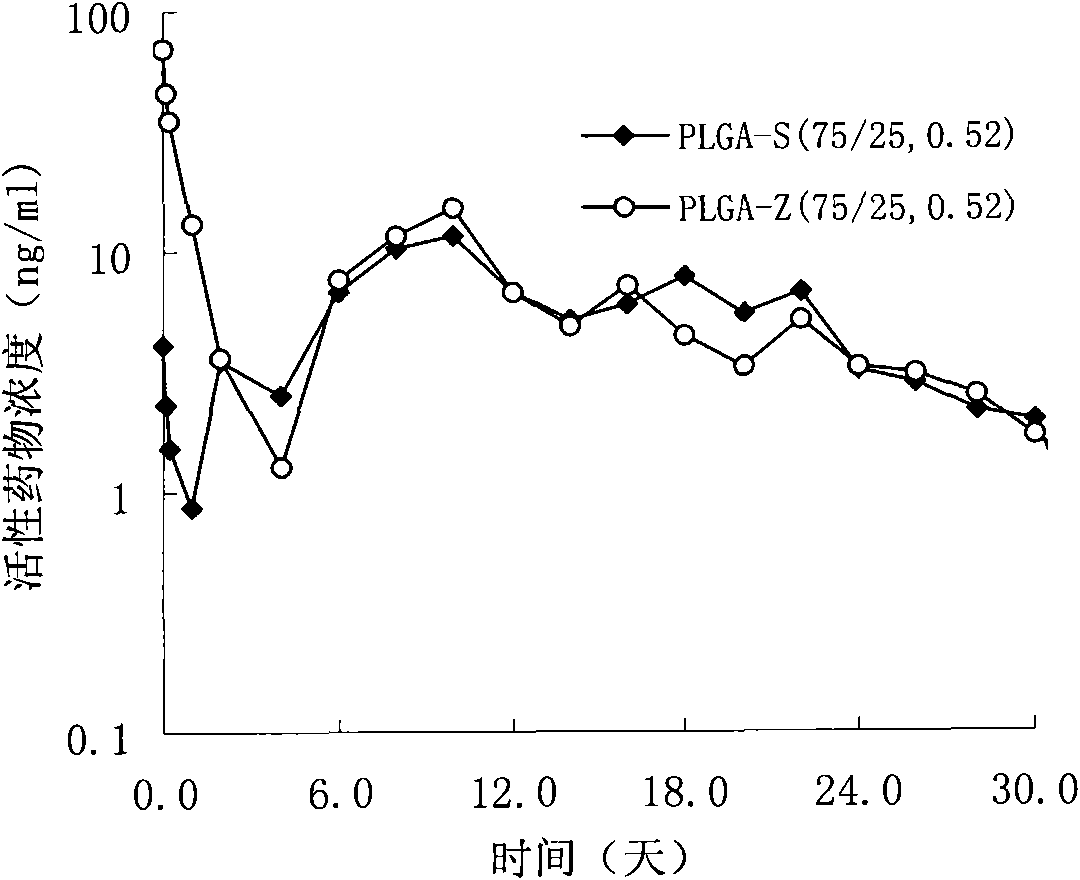
[0039] Weigh 4.0g PLGA-S (75 / 25, 0.52) molecular weight 74,000, 6.0g risperidone, add to 50ml of dichloromethane and stir to dissolve to obtain a clear solution; add this solution to a high-speed stirring cooling to 6 In 5000ml PVA solution (0.5%) at ℃, disperse and emulsify at 1000rpm for 1min, adjust the rotation speed to 300rpm, and the rotation speed of the stirring paddle is 150rpm, volatilize to remove the organic solvent, and volatilize for 6hr; filter with a sieve, wash with deionized water 5 times, freeze The powdered microspheres were dried, with a drug loading of 50.7% and an encapsulation efficiency of 84.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com