Application of dihydromyricetin in preparing medicament for preventing and treating adverse reaction of tumor chemoradiotherapy
A technology of dihydromyricetin and adverse reactions, applied in the field of medicine, can solve problems such as difficult to control and eliminate adverse reactions, macromolecular function damage, inactivation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Extraction and Purification of Dihydromyricetin from Grape Dendrobii Plant
[0022] Add 1 kg of young leaves, or shoots, or a mixture of young leaves and shoots of the Snake-toothed grape plant into water at a weight ratio of 1:8 to 1:12 (raw material: water), and soak for 30 minutes-4 After 1 hour, heat to boiling, heat-preserve and extract for 30-60 minutes, filter or centrifuge while it is hot, and collect the extract; add water again according to the weight ratio of 1:5-1:8 (raw material: water), heat to boil, and keep warm Extract for 20 to 40 minutes, filter or centrifuge while it is hot, and collect the extract; concentrate the extract to 20% to 50% of the original volume, add edible alcohol until the ethanol concentration is 40% to 75%, heat it to boiling and keep it warm for 10 ~ 60 minutes, after cooling slightly, filter or centrifuge to remove the precipitate, reclaim ethanol or directly cool the filtrate and let it stand for 1 to 2 days, collect t...
Embodiment 2
[0023] Example 2: Confirmation of the structure of dihydromyricetin
[0024] The dihydromyricetin prepared in Example 1.
[0025] (1) NMR analysis
[0026] The nuclear magnetic resonance detection instrument is an INOVA500 superconducting pulse Fourier transform nuclear magnetic resonance spectrometer.
[0027] After proton nuclear magnetic resonance spectrum, carbon spectrum, DEPT spectrum, gCOSY spectrum, gHMQC spectrum, gHMBC spectrum detection and analysis, it is determined that it is related to 3,5,7,3,,4,,5,-hexahydroxy 2,3 dihydroflavonol structure matches.
[0028] ① Hydrogen spectrum, gCOSY spectrum
[0029] Spectrum width: 6711.4Hz (~13ppm)
[0030] gCOSY spectrum: spectral width 4598.2×4598.2Hz; data points 2048×256.
[0031] Table 1, 1H NMR spectrum data and analysis
[0032] Chemical shift COZY correlation
[0033] Peak number Number of split peaks Coupling constant Number of protons Remarks
[0034] δ(ppm) bee
[0035] ...
Embodiment 3
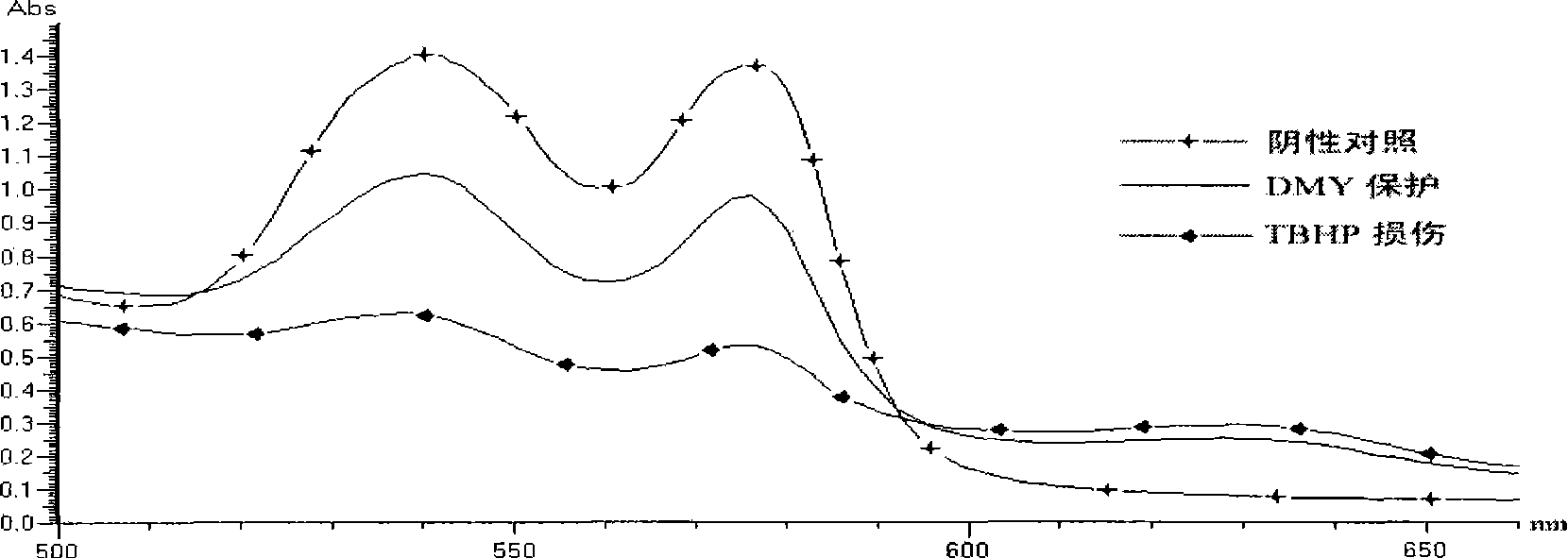
[0075] Embodiment 3: The inhibitory effect of dihydromyricetin on the formation of methemoglobin caused by tertiary peroxide (TBHP)
[0076] 1. Experimental method
[0077] Add packed erythrocytes to double distilled water to make 1% erythrocytes completely hemolyzed, add each sample component and incubate at 37°C for 30min, add TBHP to make the final concentration reach 250uM, continue to incubate for 30min, measure OD 630 Relative changes in methemoglobin content were detected. Calculation of high ferritin production inhibition rate:
[0078] Generation inhibition rate=(OD of oxidative damage group 630 - Sample group OD 630 ) / (Oxidative damage group OD 630 - control group OD 630 ).
[0079] 2. Experimental results
[0080] TBHP releases H gradually in the aqueous phase 2 o 2 , H 2 o 2 Catalyzed by free or bound iron in heme to produce superoxide anion and hydroxyl radicals through the Fenton reaction. The maximum light absorption values of the α and β peptide c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com