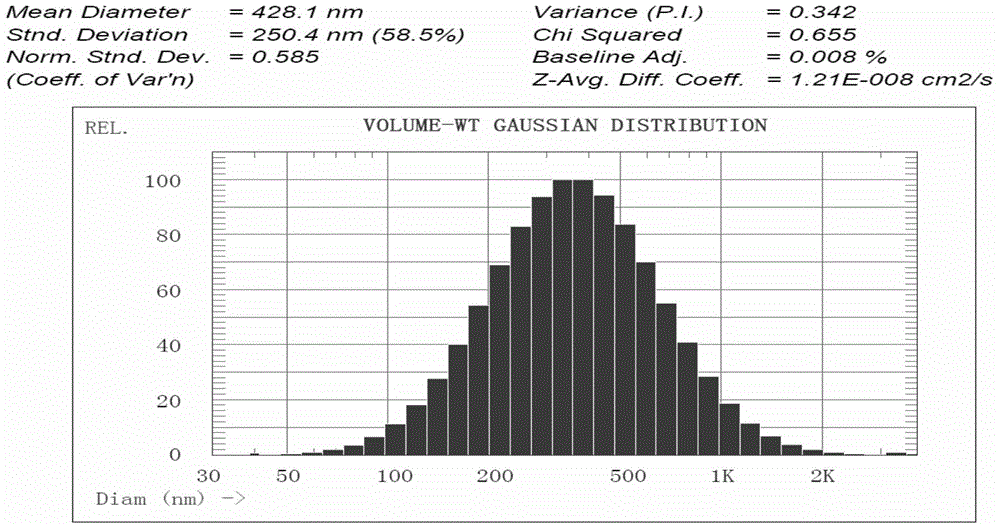
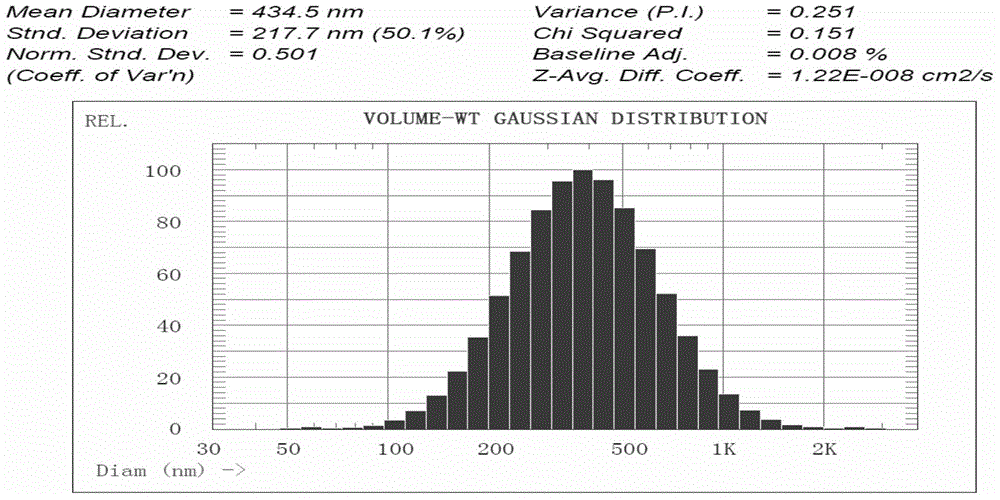
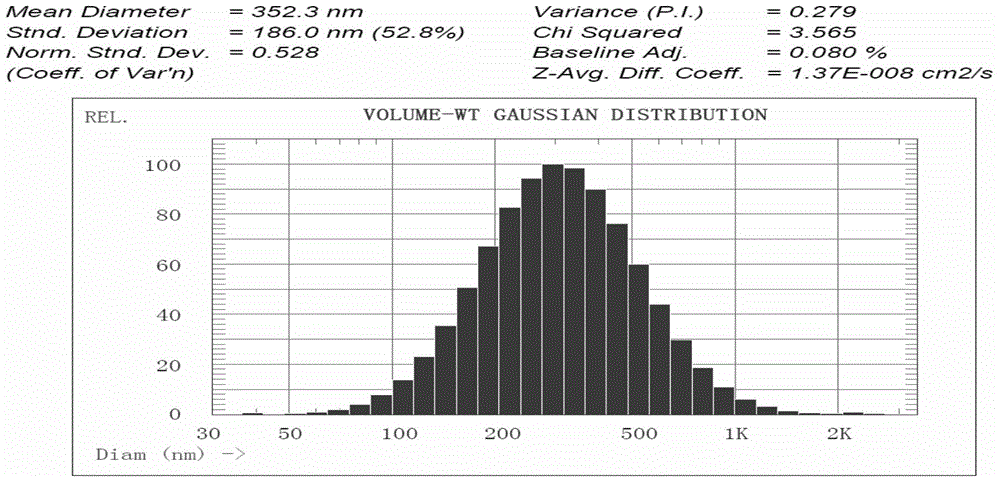
Suspension preparation of temperature change painless nano sulfadiazine metallic compound hyaluronic acid
A metal compound, sulfadiazine technology, applied in the field of sulfadiazine metal compounds, can solve the problems of difficult control of dosage, insufficient moisturizing ability of wound surface, and short duration of drug effect, so as to prolong the action time in the body, promote healing and wound healing. Restorative and healing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0190] 1. Preparation of test bacterial suspension: inoculate Escherichia coli K88 and Streptococcus strains on LB medium, incubate at 37°C for 24 hours, pick single bacteria into 3ml LB liquid medium with inoculation loop, 37°C, 200r / min Shake culture to prepare bacterial suspension;
[0191] 2. Antibacterial test sample preparation: prepare a filter paper sheet with a diameter of 7mm, sterilize it with ultraviolet radiation, and apply samples of different formulations on the filter paper sheet according to the table below under the ultra-clean workbench;
[0192] The application dosage is shown in the table below:
[0193]
[0194] 3. Comparison of antibacterial properties of samples with different particle sizes: Aseptic operation, take 100 μL of bacterial suspension and spread evenly on LB / SS plate, after standing for about 30 minutes, put a wound dressing with a diameter of 7 mm on the plate, and filter paper for antibacterial test samples Place the slices on the plat...
Embodiment 1
[0218] Preparation method: ball milling method, the preparation volume is 30g.
[0219] prescription:
[0220]
Embodiment 2
[0222] Preparation method: ball milling method, the preparation volume is 30g
[0223] prescription:
[0224]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com