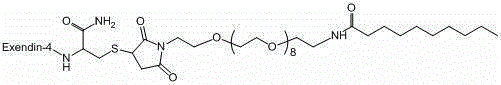Exenatide modified compound and uses thereof
A technology of exenatide and modification, applied in the field of therapeutic peptides, can solve the problems of short duration of hypoglycemia, poor clinical use effect, frequent injection, etc., and achieve long duration of hypoglycemia, long action time and stability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Exendin-4(1-39)-Cys(40)-NH 2 preparation of
[0044] .
[0045] Amino acid and its abbreviation and English abbreviation:
[0046]
[0047] The solid-phase peptide synthesis of the target peptide adopts the Fmoc method solid-phase synthesis, uses Fmoc-Rink MBHA Amide resin, uses 20% piperidine / DMF to remove Fmoc, uses HOBT / DIC as the coupling reagent, DMF as the reaction solvent, and uses Ninhydrin assay, sequentially attach the following protected amino acids to Rink MBHA Amide resin: Fmoc-Cys(Trt)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Pro-OH, Fmoc-Pro-OH, Fmoc -Pro-OH, Fmoc-Ala-OH, Fmoc-Gly-OH, Fmoc-Ser(tBu)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Pro-OH, Fmoc-Gly-OH, Fmoc-Gly -OH, Fmoc-Asn(Trt)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Leu-OH, Fmoc-Trp(Boc)-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Ile-OH , Fmoc-Phe-OH, Fmoc-Leu-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Val-OH, Fmoc-Ala-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Glu(OtBu)- OH, Fmoc-Glu(OtBu)-OH, Fmoc-Met-OH, Fmoc-Gln(Trt)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Ser(tBu)-OH, ...
Embodiment 2
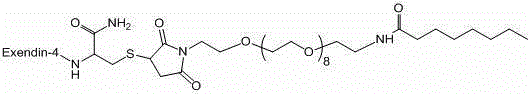
[0049] Example 2 Preparation of Compound 1
[0050]
[0051] Preparation of BP103a01
[0052] Under nitrogen protection, add 200 mL pyridine and 120 g BP103a00 (1.0eq) to a 1000ml three-neck flask, stir to cool down to 0°C, add 151.8g TsCl (1.0eq) in batches, stir for 1h, then slowly rise to room temperature, and continue stirring 3-4h. After the reaction is over, pour the reaction solution into ice dilute hydrochloric acid solution, solids are produced, add EA for extraction, wash the EA layer with dilute hydrochloric acid once, wash with saturated sodium bicarbonate, and wash with saturated brine, then anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain 119 g of crude product, and 55 g of pure BP103a01 was obtained by silica gel column chromatography.
[0053] Preparation of BP103a02
[0054] Add 55 g BP103a01 (1.0eq) and 160 mL DMSO to a 1000 mL three-neck flask, stir well, then add NaN 3 23.52g (2.0 eq), heated to 50°C for...
Embodiment 3
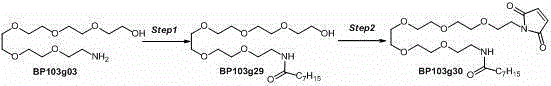
[0064] Example 3 Preparation of Compound 2
[0065]
[0066] Step1
[0067] In a 250ml three-neck flask, add 150 mL pyridine and 50 g BP103g00 (1.0eq) under nitrogen protection, stir to cool down to 0°C, add 33.7g TsCl (1.0eq) in batches, stir for 1h, slowly warm up to room temperature, and stir for 3-4h. The completion of the reaction was monitored by TLC. The reaction solution was poured into ice dilute hydrochloric acid solution, extracted with EA, and the organic phases were combined. The EA layer was washed once with dilute hydrochloric acid, saturated sodium bicarbonate, saturated brine, and anhydrous Na 2 SO 4 Drying, spinning to obtain 59g, and column chromatography to obtain 31g of pure product BP103g01.
[0068] Step2
[0069] Add 33.5g BP103g01 (1.0eq), 100mL DMSO to a 250 mL three-neck flask, stir, add 10.0 g NaN 3 (2.0 eq), heated to 50 degrees and reacted for 3 hours and cooled to room temperature. TLC monitored the completion of the reaction. The reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com