A composition containing hyaluronate and its preparation method
A technology of hyaluronic acid salt and hyaluronic acid, which is applied in the field of hyaluronic acid double gel system and its preparation, can solve the problem of high viscosity of cross-linked hyaluronic acid gel, prone to skin mounds, nodules, and low fluidity and other problems, to achieve the effect of long acting time in the body, easy to push and operate, and enhanced fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] The present invention also provides a preparation method of a hyaluronate-containing composition, the method comprising:
[0069] preparing a cross-linked hyaluronate gel using the first hyaluronic acid or hyaluronate;
[0070] using a second hyaluronic acid or hyaluronate to prepare a non-crosslinked hyaluronate gel;
[0071] The cross-linked hyaluronate gel and the non-cross-linked hyaluronate gel are mixed and then subjected to high temperature treatment to obtain a double gel system.
[0072] Specifically, the preparation of cross-linked hyaluronate gel includes:
[0073] Dissolve hyaluronic acid or hyaluronate in an alkaline solution containing a crosslinking agent, and react for 5-30 hours at 20-35°C;
[0074] Water was added to swell the gel piece and dialyzed to remove unreacted cross-linking agent and base.
[0075] Specifically, the molecular weight of the first hyaluronic acid or hyaluronate is 1 million Daltons to 2.5 million Daltons, for example, it can ...
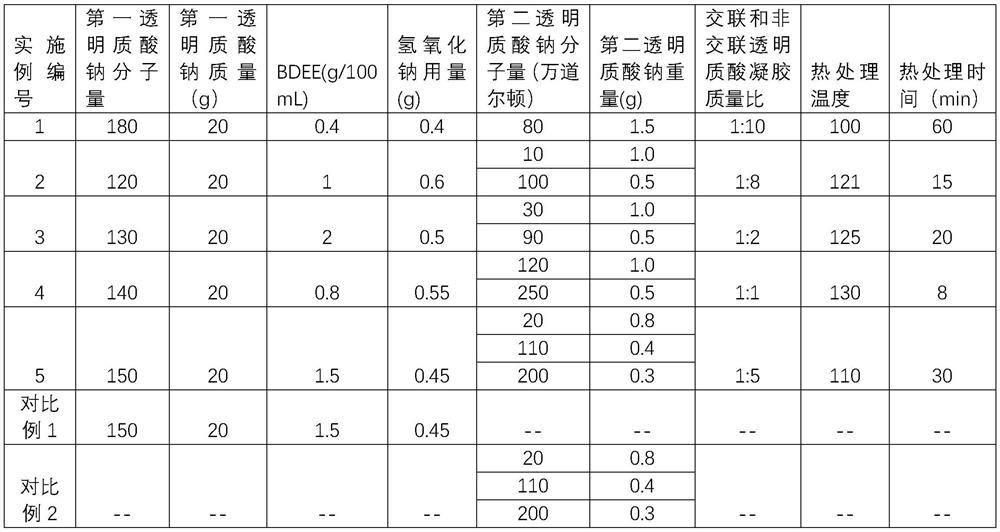
Embodiment 1
[0093] Take 20g of sodium hyaluronate with a molecular weight of 1.8 million Daltons, dissolve it in 100mL solution containing 0.4g BDDE and 0.4g sodium hydroxide, and react at 25°C for 24h; cut the gel into about 1cm 3 Granules, add phosphate buffer (concentration is 0.3mg / mL concentration, pH value is 6.0-8.0) make gel block swelling, dialyze, remove unreacted cross-linking agent and alkali, control hyaluronic acid concentration to be 1.5%, The gel has a pH value of 6.0-8.0, and passes through a 120-mesh stainless steel screen to obtain a cross-linked sodium hyaluronate gel.
[0094] Take 1.0 g of sodium hyaluronate with a molecular weight of 800,000 Daltons, add it to 100 mL of 0.5% glycerin aqueous solution, stir until dissolved, and filter through a 5 μm filter element to obtain non-crosslinked sodium hyaluronate gel with a pH of 6.0-8.0. glue.
[0095] The sodium hyaluronate gel and the non-crosslinked sodium hyaluronate gel were mixed at a mass ratio of 1:10, and after...
Embodiment 2
[0103] Take 20 g of sodium hyaluronate with a molecular weight of 1.2 million Daltons, dissolve it in 100 mL of a solution containing 1.0 g of BDDE and 0.6 g of sodium hydroxide, and react at 25° C. for 24 hours. Cut the gel into about 1cm 3 Granules, add phosphate buffer (concentration is 0.3mg / mL, pH value is 6.5) to make the gel piece swell, remove unreacted cross-linking agent and alkali through dialysis 3-6 times, control the hyaluronic acid concentration to be 1.5 %, the pH value of the gel is 6.0-8.0, and passed through a 120-mesh stainless steel screen to obtain a cross-linked sodium hyaluronate gel.
[0104] Take 1.0 g of sodium hyaluronate with a molecular weight of 100,000 daltons and 0.5 g of sodium hyaluronate with a molecular weight of 1 million daltons, add them to 100 mL of 0.5% glycerin aqueous solution, stir until dissolved, and filter through a 5 μm filter element to obtain pH 6.0-8.0 non-cross-linked sodium hyaluronate gel.
[0105] The sodium hyaluronate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com