Anti-microbial fusion proteins and application thereof
A technology of fusion protein and antibacterial protein, which is applied in the field of biotechnology and genetic engineering, can solve the problems of antibacterial peptides with narrow antibacterial spectrum, easy to be metabolized out of the body, and unsatisfactory bactericidal effect, so as to enhance the bactericidal effect, improve the expression level, and prolong the antibacterial peptide in vivo. The effect of action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Construction of expression vector
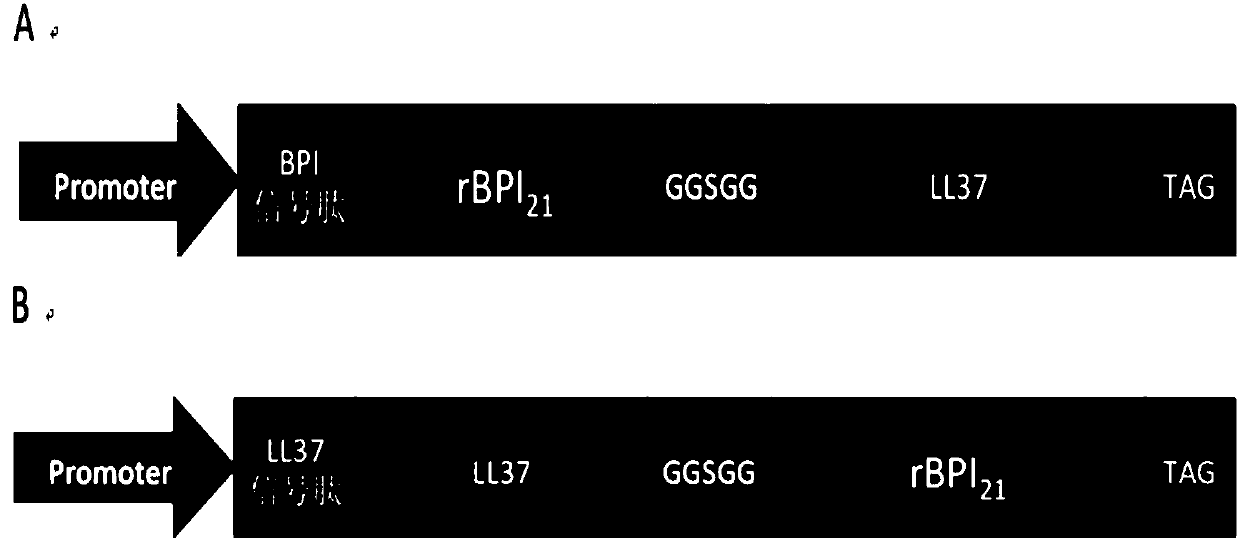
[0038] According to the amino acid sequence of the antibacterial fusion protein and the preferred codon of Chinese hamster, the fusion protein gene was designed and synthesized, and the synthetic gene was cloned into the pLVX-Tight-Puro vector to construct the fusion protein expression vector. Finally, two forms of fusion protein gene expression vectors, BPI-LL37 or LL37-BPI21, were obtained. The difference between the two expression vectors is that the order of fusion is different, and the signal peptide of each protein located at the N' end guides the exocrine expression of the entire fusion protein. The schematic diagram of the construction of the vector is as follows figure 1 as shown, figure 1 A is the schematic diagram of the vector structure of pLVX-rBPI21-LL-37, figure 1 B is the structural representation of pLVX-LL-37-rBPI21 vector.
Embodiment 2
[0039] Example 2 Protein expression of expression vector
[0040] (1) The antibacterial peptide LL-37 and its signal peptide protein coding gene sequence, the GGSGG flexible peptide coding gene sequence, rBPI21 and its signal peptide protein coding gene sequence were obtained by artificial synthesis; at the same time, in order to enhance the expression level of the fusion protein, According to the preferred codon theory, the rare codons on the LL-37 gene and rBPI21 gene were replaced with the preferred codons of Chinese hamsters to optimize the gene sequence of the fusion protein. The encoded nucleotide sequences of optimized LL-37 and rBPI21 are respectively shown in SEQ ID NO: 10 and SEQ ID NO: 8
[0041] (2) Carry out single and double enzyme digestion identification on the constructed vector
[0042] When we designed and constructed the pLVX-LL-37-rBPI21 and pLVX-rBPI21-LL-37 vectors, we added BamHI and MluI restriction sites at the 5' end and 3' end of the target gene re...
Embodiment 3
[0046] Example 3 Exocrine expression of fusion protein in CHO cells
[0047] (1) Cell culture
[0048] Chinese hamster ovary cells (CHO cells) were cultured in DMEM / F12 medium containing 10% FBS at 37°C, 5% CO 2 Conditioned culture, cells grow to 70%-80% density, ready for transfection.
[0049] (2) Cell transfection by liposome method
[0050] Replace with fresh medium 1 hour before transfection. Referring to Table 2 below, dilute plasmid DNA and liposome transfection reagent in two centrifuge tubes with serum-free DMEM / F12 medium. Gently invert the centrifuge tube 3-4 times to mix the liquid.
[0051] Immediately add the diluted liposomes to the diluted plasmid DNA solution, vortex immediately or invert the centrifuge tube 3-4 times to mix the liquid evenly. Stand at room temperature for 20 minutes to form the liposome / DNA complex, and the standing time should not exceed 30 minutes. Add all the liposome / DNA complexes into the cell culture medium, shake the culture dish...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com