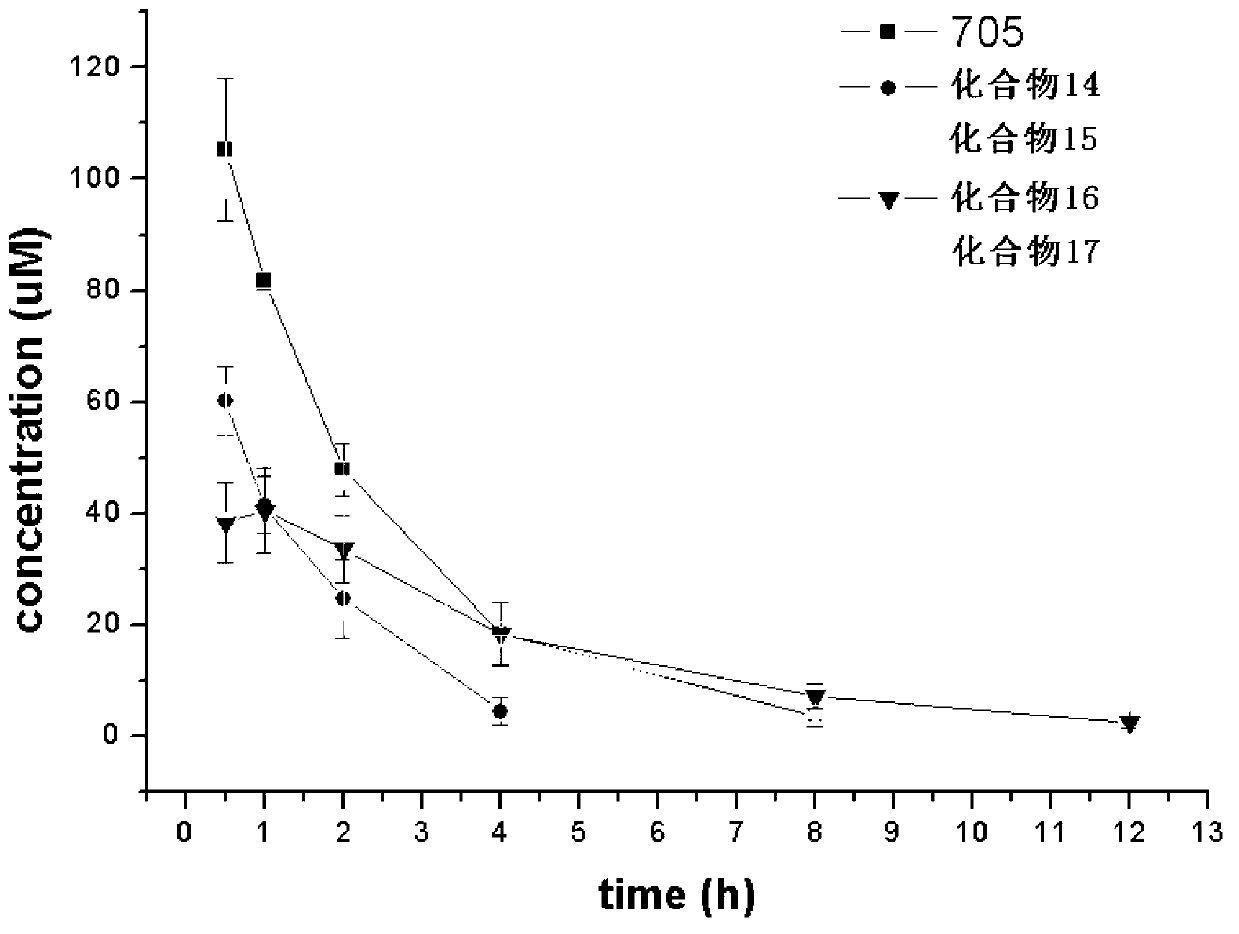
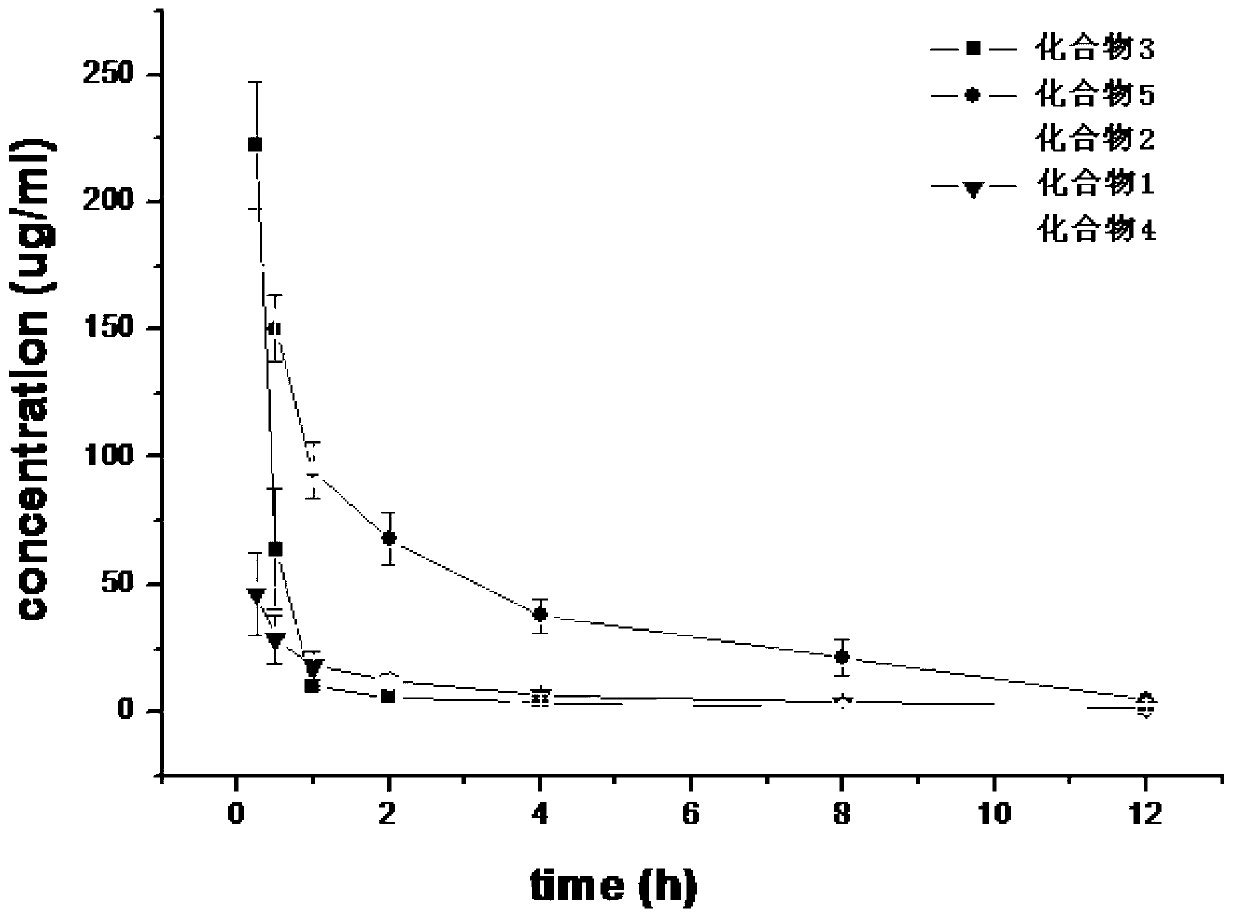
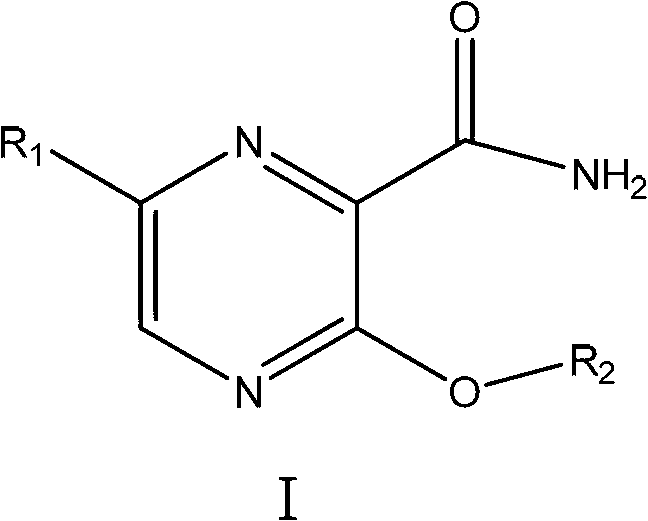
Preparation method and purpose of 3-alkoxy-substituent-2-pyrazinyl formamide compounds
A technology for pyrazinecarboxamide and compounds is applied in the field of preparing antiviral drugs, and can solve the problems of short half-life, poor pharmacokinetic properties, adverse drug effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0064] Example 1 Preparation of 3-(n-propyloxy)-2-pyrazinecarboxamide (Compound 1) (Method 1).
[0065]
[0066] In a dry three-necked flask with a thermometer, condenser and drying tube, add 30mL of anhydrous propanol, add 146mg (6.34mmol) of sodium, stir at room temperature or slightly heat until the sodium particles disappear completely, add the purified 3- Chloro-2-pyrazinecarboxamide 500mg (3.17mmol), heated to 80°C, reacted for 20min, cooled to room temperature, added 1mL of glacial acetic acid, concentrated under reduced pressure to dryness, mixed with silica gel and separated by Flash chromatography column (two Methyl chloride / methanol elution) to obtain the target compound, 1 HNMR (CDCl 3 )Δ1.50(3H,t,J=7.2Hz),
[0067] 4.58(2H,q,J=7.2Hz), 6.07(1H,brs), 7.62(1H,brs), 8.26(1H,s), 8.28(1H,s);
[0068] EIMS(m / e,100),167(M + ,25),150(60),123(35),96(40),80(40),68(100).
Embodiment 23
[0069] Example 2 Preparation of 3-(n-propyloxy)-2-pyrazinecarboxamide (Compound 1) (Method 2).
[0070] step 1 Preparation of 3-(n-propyloxy)-2-pyrazinecarbonitrile
[0071]
[0072] In a dry three-necked flask with thermometer, condenser and drying tube, add 10 mL of anhydrous DMF, add 3-chloro-pyrazine-2-carbonitrile (978 mg, 7.00 mmol), stir to dissolve, add anhydrous n-propyl Alcohol (841mg, 14mmol) and anhydrous triethylamine (1.5ml, about 11.85mmol), warm up to 80-120C and react for 2 hours, after cooling to room temperature, dilute with water, extract with ethyl acetate, wash with water, and wash with sodium chloride solution , Concentrate to dryness under reduced pressure to obtain the crude product without separation, and use it directly in the next reaction after drying.
[0073] Step 2 Preparation of 3-(n-propyloxy)-2-pyrazinecarboxamide (Compound 1)
[0074]
[0075] In a three-necked flask with a thermometer, add 3-(n-propyloxy)-2-pyrazinecarbonitrile (980mg, about 6m...
Embodiment 3
[0077] Example 3 Preparation of 3-(isopropyloxy)-2-pyrazinecarboxamide (compound 2)
[0078] The method is the same as in Example 1, except that isopropanol is used instead of n-propanol to obtain the title compound. 1 HNMR (CDCl 3 )Δ1.45(6H,d,J=6.0Hz), 5.50(2H,t,J=7.2Hz), 6.14(1H,brs),7.69(1H,brs), 8.27(1H,d,J=2.4 Hz),8.29(1H,d,J=2.4Hz); ESIMS(m / e)182(MH + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com