Sphaelactone dimethylamine lipidosome atomizing inhalant and application thereof
A dimethylamine ester and liposome technology, applied in the treatment of pulmonary fibrosis, in the field of laughing lactone-containing dimethylamine aerosol inhalation, can solve the problems of pulmonary fibrosis without specific drugs and difficult to cure, etc. The effect of prolonging the action time in vivo, improving the binding rate and changing the pharmacokinetic characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Preparation of PSMC
[0055] Experimental method and procedure: Dissolve egg yolk lecithin and cholesterol in ethanol at a ratio of 5:1, evaporate under reduced pressure at 50°C on a rotary evaporator, and evaporate the solvent to a uniform film. Dissolve the weighed ACT001 in PBS and preheat it to 60°C, add it to the spun membrane, hydrate the membrane, wash the membrane with a probe, and filter with a microporous membrane to obtain a PSMC ACT001 suspension.
Embodiment 2
[0056] Example 2 Establishment of mouse lung fibrosis model and ACT001 pharmacodynamic test
[0057] Experimental methods and steps:
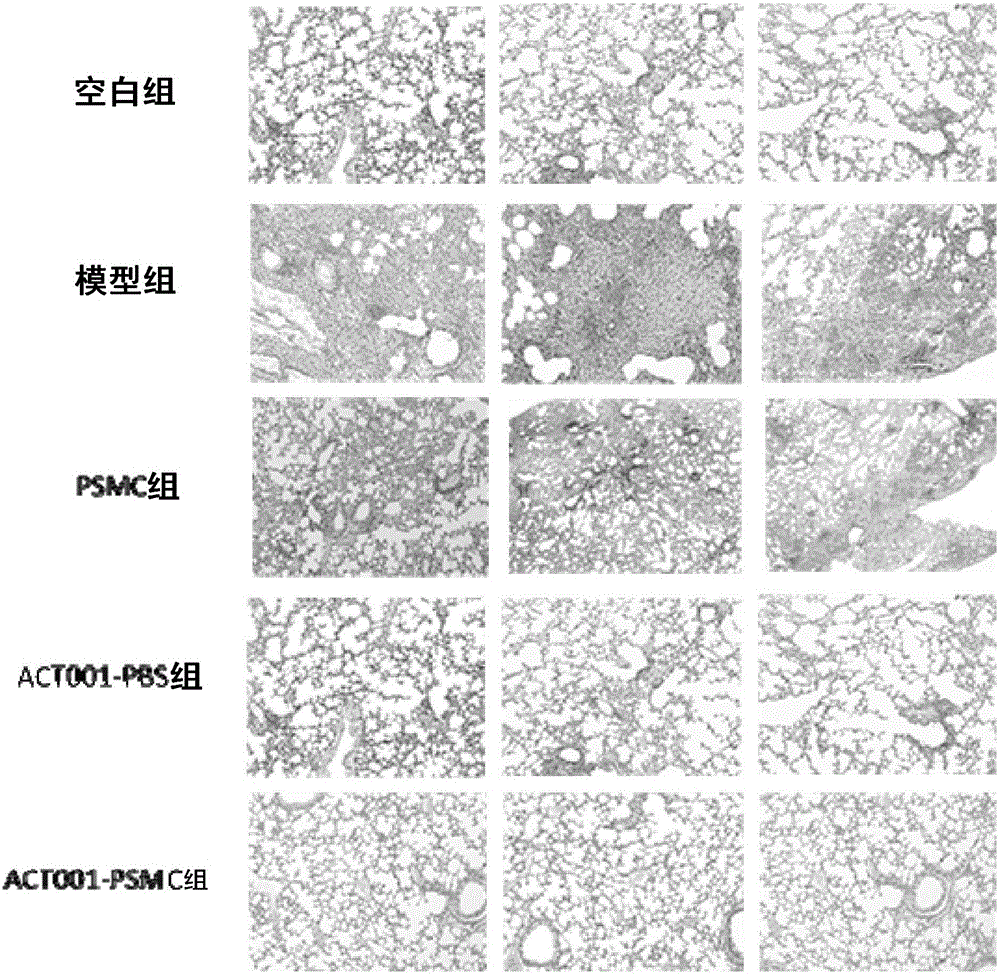
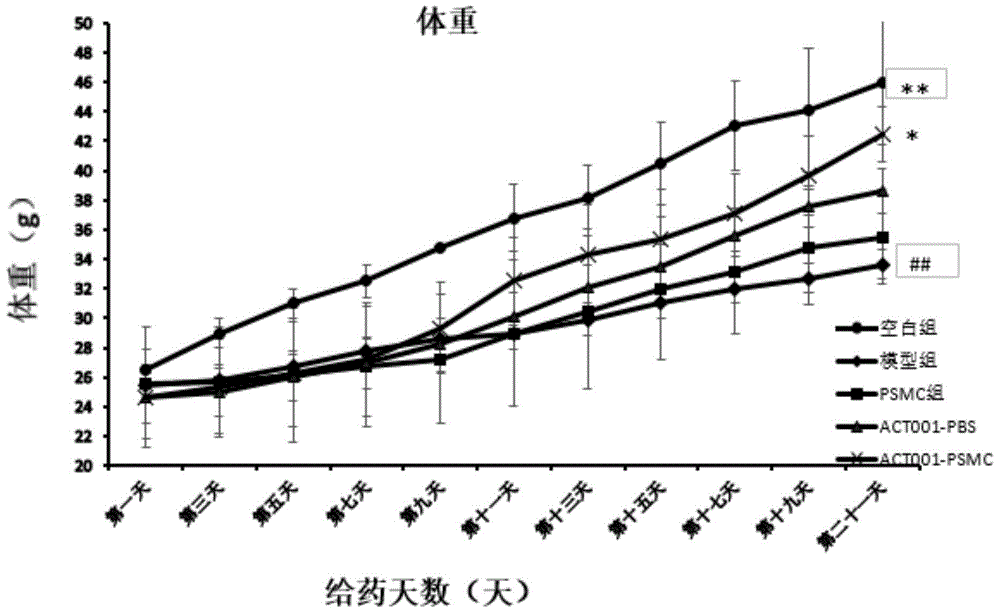
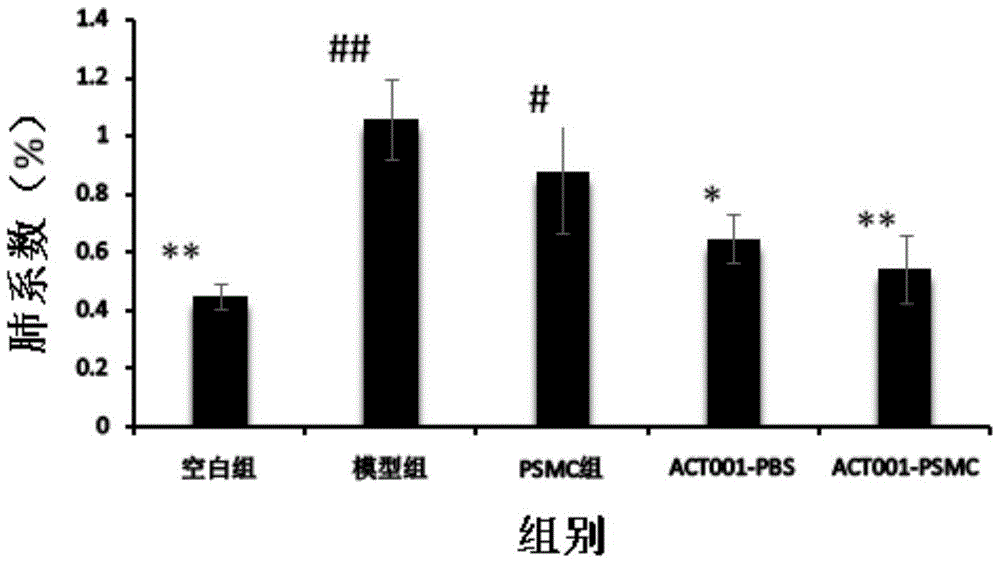
[0058] 1. Establishment of mouse pulmonary fibrosis model: 25 mice were randomly divided into five groups, blank group, model group (pulmonary fibrosis model group), blank PSMC group (blank PSMC after modeling), ACT001-PBS Group (administer PBS solution after modeling), ACT001-PSMC group (administer PSMC after modeling), 5 animals in each group. In the blank group, 0.15 mL of saline solution was intragastrically administered once; in the other groups, 0.15 mL of bleomycin solution was administered to the lung.
[0059] 2. Administration and treatment of pulmonary fibrosis mice: In the second week after modeling, the mice were given drug treatment. The blank group and the model group were perfused with 0.1mL PBS each time (simulating clinical aerosol inhalation administration form) The blank PSMC group was perfused with 0.1 mL of blank PSMC solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com