4-(1-phenethyl)-1,3-dihydroxy benzene liposome and preparation method thereof
A technology of hydroxyphenyl ester and dihydroxybenzene, which is applied in the field of 4--1 to achieve the effect of improving stability and prolonging the action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of 4-(1-phenylethyl)-1,3-dihydroxyphenyl liposomes
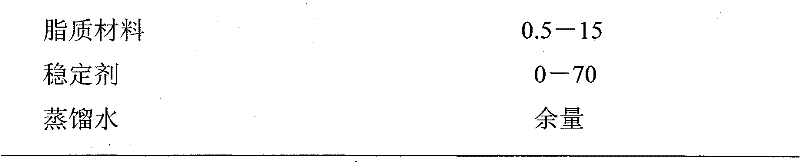
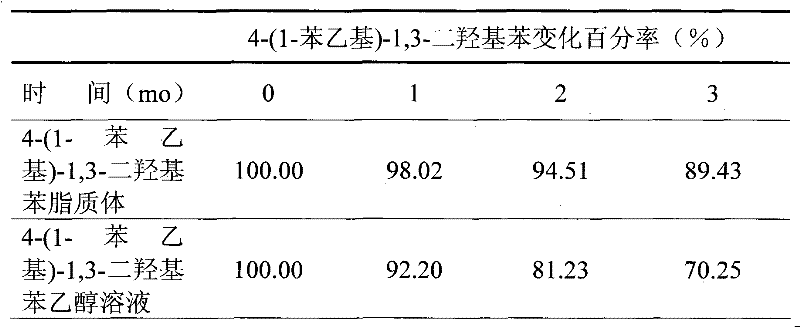
[0026] Get 4-(1-phenylethyl)-1,3-dihydroxybenzene 1g, soybean lecithin 3.8g, cholesterol 1.2g, put in the round bottom flask of 1000ml, add 50ml chloroform and methanol mixed solution (chloroform:methanol =1:1), dissolved at 55°C, placed in a constant temperature water bath at 55°C for rotary film evaporation to form a lipid film; then add 994ml of distilled water preheated to 55°C, hydrate, shake, and obtain colostrum; colostrum Perform secondary emulsification with a high-pressure homogenizer to obtain 1000 g of 4-(1-phenylethyl)-1,3-dihydroxyphenyl liposome.
Embodiment 2
[0027] Embodiment 2: Preparation of 4-(1-phenylethyl)-1,3-dihydroxyphenyl liposome
[0028] Take 80g of 4-(1-phenylethyl)-1,3-dihydroxybenzene, 20g of soybean lecithin, and dissolve it with 100ml of ethanol under heating in a water bath at 75°C to obtain a lipid solution; take 700g of glycerin and 100ml of distilled water, Mix well, and heat in a water bath to 75°C to obtain an aqueous phase. Inject the lipid solution into the water phase at 75°C and shake evenly to obtain colostrum. Place the colostrum in a high-pressure homogenizer for secondary emulsification to obtain 1000 g of 4-(1-phenylethyl)-1,3-dihydroxyphenyl liposome.
Embodiment 3
[0029] Example 3: Preparation of 4-(1-phenylethyl)-1,3-dihydroxyphenyl liposomes
[0030] Take 100g of 4-(1-phenylethyl)-1,3-dihydroxybenzene, 50g of egg yolk lecithin, 50g of cholesterol, and 20g of ceramide in a spinner bottle, add 200ml of dichloromethane, and dissolve it under heating at 50°C , placed in a constant temperature water bath at 50°C to rotate the film to evaporate to form a lipid film; then add 780ml of distilled water preheated to 65°C, hydrate, shake, and obtain colostrum; use ultrasonic emulsification to perform secondary emulsification on the colostrum. 1000 g of 4-(1-phenylethyl)-1,3-dihydroxyphenyl liposome was obtained.
[0031] Example 4: Preparation of 4-(1-phenylethyl)-1,3-dihydroxyphenyl liposomes
[0032] Take 30g of 4-(1-phenylethyl)-1,3-dihydroxybenzene, 30g of distearoylphosphatidylcholine, 50g of dipalmitoylphosphatidylcholine, and 10g of cholesterol, and heat it in a water bath at 70°C with 200ml of ethanol Dissolve it under pressure to obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com